Patients With Small Cell Lung Cancer Experience Tumor Response With Liposomal Irinotecan
Findings from part 1 of the phase II/III RESILIENT trial of irinotecan hydrochloride liposome injection in second-line treatment of small cell lung cancer showed that for close to 50% of patients, disease control was maintained at 12 weeks. These data were presented at the 2019 World Conference on Lung Cancer.
Luis G. Paz-Ares, MD, PhD

Luis G. Paz-Ares, MD, PhD
Findings from part 1 of the phase II/III RESILIENT trial of irinotecan hydrochloride liposome (Onivyde) injection in second-line treatment of small cell lung cancer (SCLC) showed that for close to 50% of patients, disease control was maintained at 12 weeks. These data were presented at the 2019 World Conference on Lung Cancer.1,2
The liposomal irinotecan induced an objective response rate (ORR) of 44% among 25 patients treated with the optimal dose of 70 mg/m2. The ORR comprised 11 partial responses (PRs). The rate of best overall response, which included all PRs as well as patients with stable disease, was 72%. The 12-week disease control rate, which included lasting PRs and instances of stable disease, was 48%. Sixty-eight percent of patients had some level of tumor shrinkage.1
“Immunotherapies and combination therapies have proven beneficial in the first-line setting, but despite these advances, many patients with SCLC rapidly relapse due to the aggressive nature of the disease,” Luis G. Paz-Ares, MD, PhD, lead investigator and chief physician at Hospital Universitario 12 de Octubre in Madrid, Spain, said in a press release.
“While the current standard of care in the second-line setting can extend survival, treatment toxicity has prevented some patients from receiving the full recommended dose. There is a clear need for more treatment options that may give more patients the chance to remain on therapy. It is positive that the RESILIENT trial will continue to investigate this,” added Paz-Ares.
The 2-part, open-label phase II/III RESILIENT study is exploring single-agent liposomal irinotecan in patients with SCLC with disease progression following a frontline platinum-based regimen. Part 1 of the trial involves dose-finding and dose-escalation analyses, and in part 2, which has just started, patients are being randomized to compare the efficacy of liposomal irinotecan to topotecan (Hycamtin), the current standard of care. Key endpoints for part 2 are progression-free survival and overall survival.
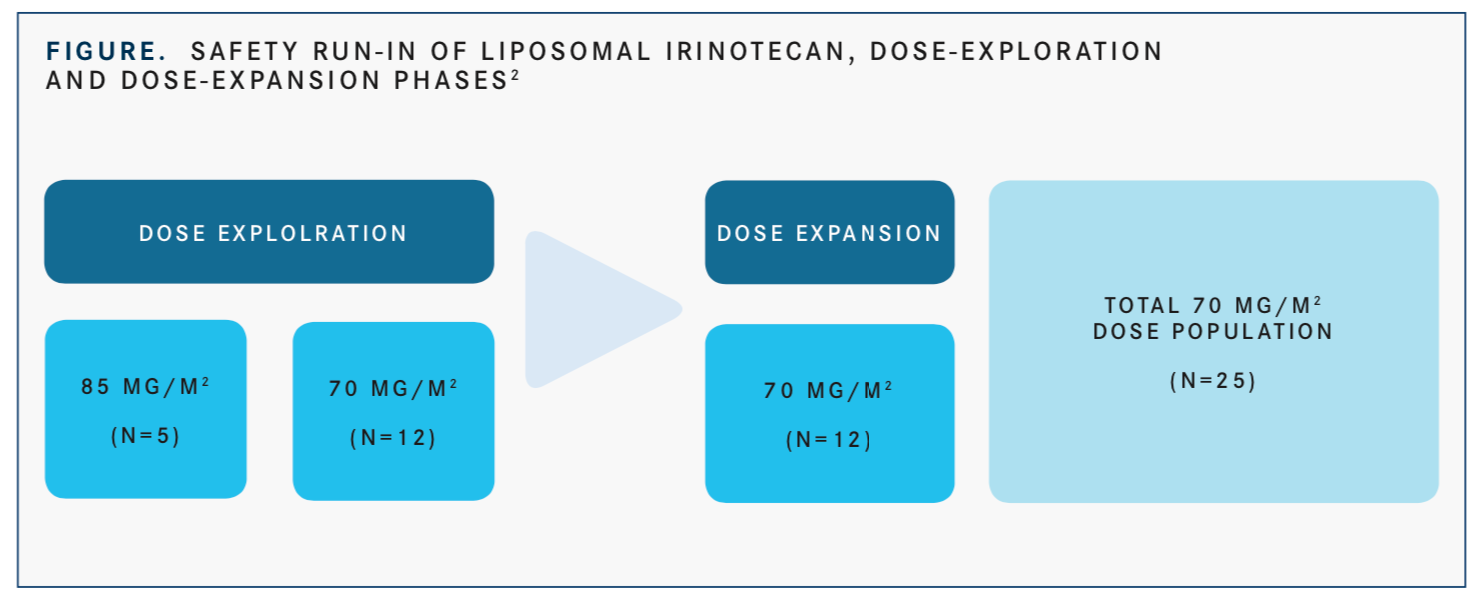
Part 1 data from 30 patients with a median age of 60 years (range, 48-73) were presented at the WCLC. In the dose-finding phase, patients received liposomal irino- tecan every 2 weeks at 70 mg/m2 or 85 mg/m2. However, after 5 patients were enrolled to receive the 85 mg/m2 dose, investigators determined that the 85 mg/m2 dose was not tolerable because of dose-limiting toxicities they observed. During this phase, 12 patients received the 70 mg/m2 dose, which investigators deemed tolerable. This led to an additional 13 patients being treated at 70 mg/m2 in the dose-expansion phase (FIGURE).2
Among the 25 patients who received the 70 mg/m2 dose, 40% (n = 10) were male, the median age was 59.8 years, and 88% had an ECOG performance status of 1. The median time since most recent disease progression was 3.2 weeks, 2 patients had locally advanced disease, and 23 patients had metastatic disease. Twelve patients had completed the study, 7 remained in the study, and 6 patients had died. Two patient deaths resulted from adverse events related to the study drug.
Grade ≥3 treatment-emergent adverse events (TEAEs) occurred in 40% (n = 10) of patients treated at 70 mg/m2. The most common grade 3 gastrointestinal TEAE was diarrhea (n = 5). Grade ≥3 hematologic TEAEs included neutropenia (n = 4), anemia (n = 2), and thrombocytopenia (n = 2). There was also one case of grade ≥3 fatigue.
Liposomal irinotecan is a nanoliposomal encapsulation of irinotecan. The liposomal formulation allows the irinotecan to remain in circulation longer than standard irinotecan does. Additionally, this mechanism allows for higher drug uptake within tumor cells and greater conversion of irinotecan to its active form,3SN-38.
The FDA approved liposomal irinotecan in October 2015 for use in combination with 5-fluorouracil (5-FU) and leucovorin as a treatment for patients with meta- static pancreatic cancer following administration of a gemcitabine-based regimen.4
“Onivyde has been proven to help many metastatic pancreatic cancer patients whose disease has progressed following gemcitabine-based therapy to live longer,” Yan Moore, MD, senior vice president and head of oncology therapeutic area at Ipsen, Paris, France, which develops liposomal irinotecan along with Servier, said in a press release. “By applying this research to other hard-to-treat-cancers, like SCLC, we aim to evaluate the potential benefit investigational Onivyde may bring to patients who otherwise would have limit- ed treatment options.”
References
- Ipsen and Servier announce initial phase II/III clinical data evaluating investigational liposomal irinotecan (Onivyde) as a second-line treatment for small cell lung cancer (SCLC) at the International Associations for the Study of Lung Cancer 2019 world conference on lung cancer [news release]. Paris, France: Ipsen; September 8, 2019. bit.ly/2lPeuHL. Accessed September 9, 2019
- Paz-Ares L, Spigel D, Chen Y, et al. Initial efficacy and safety results of irinotecan liposome injection (NAL-IRI) in patients with small cell lung cancer [Abstract OA03.03]. Presented at: International Association for the Study of Lung Cancer 20th World Conference on Lung Cancer; September 7-10, 2019; Barcelona, Spain.
- Zhang H. Onivyde for the therapy of multiple solid tumors. Onco Targets Ther. 2016;9:3001-3007. doi: 10.2147/OTT.S105587.
- Simon S. FDA approves Onivyde for advanced pancreatic cancer. American Cancer Society website. bit.ly/2vApadL. Published October 22, 2015. Accessed September 19, 2019.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More