Participants Discuss Treating a Patient With Myelofibrosis and Anemia
During a Targeted Oncology™ Case-Based Roundtable™ event, Salman Fazal, MD, discussed with participants which symptoms of myelofibrosis impact their quality of life and how they would treat a patient experiencing anemia.
Salman Fazal, MD (MODERATOR)
Director
Cell Transplantation Program
Division of Hematology & Cellular Therapy
Allegheny Health Network
Pittsburgh, PA

EVENT REGION New Jersey and Pennsylvania
PARTICIPANT LIST Abhirami Vivekanandarajah, MD | Hayan Moualla, MD | Subhash C. Proothi, MD | Meher Burki, MD | Simi Masand Rai, MD | Rajesh Thirumaran, MD | Nirmala Nathan, MD
CASE SUMMARY
A 76-year-old woman presented to her physician with symptoms of mild fatigue, night sweats, and abdominal pain/fullness for 4 months; she also reported an unexplained weight loss of 12 lb. Her spleen was palpable 8 cm below the left costal margin. She had no known comorbidities.
Laboratory values included a red blood cell count of 3.40 × 106/μL, hemoglobin of 9.8 g/dL, and a platelet count of 181 × 103/μL. Next-generation sequencing showed a JAK2 V617F mutation. Her karyotype was 46,XX. A bone marrow biopsy showed megakaryocyte proliferation and atypia with evidence of reticulin fibrosis. The patient received a diagnosis of primary myelofibrosis with a high-risk result from the Dynamic International Prognostic Scoring System, an intermediate risk result from the Mutation-Enhanced International Prognostic Score System (MIPSS70), and a high risk result from the MIPSS70+ version 2.0.
DISCUSSION QUESTION
- In your experience with myelofibrosis, which symptoms/presentations have the most negative impact on patients’ quality of life?
FAZAL: I want to get input from all our participants about their experience with myelofibrosis, and which symptoms and presentations have the most negative impact on patients’ quality of life.
VIVEKANANDARAJAH: These patients are usually very fatigued; they have abdominal fullness, early satiety usually due to a big spleen. Those are the most important symptoms and complaints that we hear from patients.
FAZAL: Do you usually incorporate any tools to assess their symptoms? How do you capture symptoms?
VIVEKANANDARAJAH: I know there are tools and questionnaires. I just ask them [questions in a] clinical assessment on how they feel and what affects their quality of life.
MOUALLA: Just the review of symptoms; I agree with what Dr Vivekanandarajah said. Those are the most common symptoms, the fatigue and bloating.
PROOTHI: [The most common symptoms I see are] anemia and the splenomegaly.
FAZAL: Do you think that [those symptoms] usually caused most of the problems for the patients that you see in your practice?
PROOTHI: Not all the symptoms. The constitutional symptoms may not be from that. [They may be] just from the disease alone.
FAZAL: Do you use the Myeloproliferative Neoplasm Symptom Assessment Form, as well, to assess the symptoms present at the time of diagnosis?
MOUALLA: Just for clinical trials.
FAZAL: It is present in the National Comprehensive Cancer Network [NCCN] guidelines, and it could be considered for part of the evaluation [process] to get a key sense about the disease.1 We do have use for it in clinical practice, in addition…it was used in the clinical trial data, too.
DISCUSSION QUESTIONS
- In your practice:
- What is the trigger to initiate therapy for a patient with myelofibrosis?
- How important is it to initiate therapy early?
- When do you consider clinical trial enrollment?
FAZAL: In your practice, what is the timing to start JAK [Janus kinase] inhibitor therapy? How do you choose? How does the nature and the burden of symptoms influence your decision to initiate JAK inhibitor therapy?
BURKI: I start prescribing it when I feel that constitutional symptoms are affecting the patient’s quality of life. My most recent patient was having drenching night sweats and a lot of fatigue. His spleen was so big that [it felt] like a football, and he had a very interesting presentation of polycythemia vera transforming into myelofibrosis with a marrow that was not that convincing for myelofibrosis, but [was for] the other symptoms. I started him on pacritinib [Vonjo] because his platelet count was low, in the 40,000/μL range.
FAZAL: Do you use the IPSS or prognostic models to determine whether the patient is going to be a candidate for therapy? Or is it mainly based on the symptoms or the presentation?
RAI: I think the cytopenias—the anemia or the thrombo-cytopenia—[can help determine therapy]. I still try to use hydroxyurea or an erythropoiesis-stimulating agent [ESA] when I can after initial diagnosis if [the patient is] not that symptomatic or they don’t have spleen [symptoms]. But once they start experiencing weight loss, early satiety, a big spleen, and start becoming symptomatic, then I usually [choose treatment]. I don’t typically use the scoring system. It’s usually 20 minutes in and out with patients [who are not symptomatic]. But if they start becoming symptomatic, then I jump in. Like you said, if the platelet count is low or the platelet count is above 50,000/μL, then I can decide which drug I want to use.
FAZAL: Of the nonspleen symptoms, is there anything that would trigger the need to start therapy?
RAI: If the [blood or platelet] counts are dropping, or [the patient is] on ESA and now it’s not helping anymore.
MOUALLA: I start JAK inhibition as soon as a myelofibrosis diagnosis is made. I think a few years ago there were some data that suggested there might be an improvement in survival with early ruxolitinib use.2
PROOTHI: I generally wait for the symptoms.
FAZAL: In terms of the blood counts, is there a blood count that is a trigger? Do you get worried about [using] anemia or thrombocytopenia to decide about initiating therapy?
PROOTHI: If the hemoglobin is less than 9 g/dL in men and less than 8 g/dL in women, and the platelet count is less than 100,000/μL, that’s a trigger.
FAZAL: When do you consider clinical trial enrollment? Do you have a center close by where you refer patients for evaluation or clinical trial enrollment?
THIRUMARAN: I would consider patients…50 years [or younger for clinical trial].
PROOTHI: I’ll [evaluate whether] they are a candidate for transplant.
RAI: If they are younger and more fit, [I think about transplant for that patient].
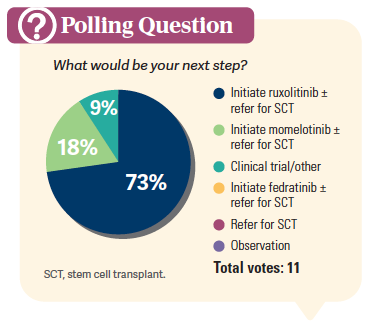
FAZAL: In the NCCN recommendation in terms of the management of high-risk myelofibrosis…patients would be considered a candidate for clinical trial, ruxolitinib, or fedratinib [Inrebic].1 That is a category 1 recommendation. Pacritinib up front is a category 2B recommendation, and if the patient has no response or a loss of response, then you can consider an alternative JAK inhibition that has not been used before, or a clinical trial.
We know there are some challenges in terms of each of these JAK inhibitors. Cytopenias are a challenge with ruxolitinib. With fedratinib, in addition to gastrointestinal toxicity, there have been concerns [about] encephalopathy. Hemorrhage and cardiovascular issues [are seen] with pacritinib. Momelotinib [Ojjaara] has adverse events that could be associated with any JAK inhibitor. We do monitor blood counts during therapy and avoid abrupt discontinuation with ruxolitinib because it could cause a flare-up of the symptoms or even rebound symptoms.
PROOTHI: This person is 76 years old. She has no comorbid conditions. Is she a candidate for transplant because there’s no comorbid condition, or is the age a factor?
FAZAL: Age is a factor, certainly. I think transplant is a challenge for myelofibrosis. As a transplanter, it always plays a role. For any patient diagnosed with myelofibrosis, starting them on a JAK inhibitor certainly optimizes the patient’s condition. In myelofibrosis, it’s more relevant that once you start them, you can fully assess their clinical condition. Because let’s say, for example, she has lost weight, even a 12-lb weight loss, and she had constitutional symptoms. She is not going to [seem like a good candidate] when she walks into the transplant office. But a few months into therapy her disease may look very different. At this point in time, it’s in her best interest to start a JAK inhibitor. Whether she is transplant eligible or not, age will play some role.
CASE UPDATE
The patient was not interested in a transplant. A decision was made to initiate momelotinib due to moderate anemia.
DISCUSSION QUESTIONS
- What are the treatment goals for a patient like this?
- If this patient was referred for transplant evaluation, what first-line therapy would you have chosen?
MOUALLA: The anemia wasn’t severe, and one can possibly try an ESA if she’s on a JAK inhibitor with the possible worsening of hemoglobin [levels]. The main thing would be [targeting the] symptoms, and if [the patient is] transplant eligible, get them to a cure, but that’s not my call.
PROOTHI: I would stick to momelotinib because the only thing she has blood count–wise was the anemia.
FAZAL: The other question is if this patient was referred for transplant evaluation, would that have made a difference in terms of which JAK inhibitor would have been used?
PROOTHI: No, but most of the studies done were with ruxolitinib, and there was no difference whether they used it up front before the transplant or not. I don’t think there are much data for momelotinib.
FAZAL: There are no data for patients going to transplant just with momelotinib. That’s relevant. The response to the JAK inhibitors is very important in terms of incorporating ESAs. Even if someone is anemic or if they have an enlarged spleen, they tend to have progressive splenomegaly on the ESAs. Once they’re on a JAK inhibitor when the spleen is not an issue, then they tend to tolerate [ESAs]. How many of you would have referred this patient for transplant evaluation?
I would mention what transplant [involves] to see whether she would be interested in that treatment.
RAI: Are you using fedratinib at all? There were some concerns about the encephalopathy on those trials.
FAZAL: Yes, I have patients on fedratinib as well. You could use that in certain patients. I did participate in the FREEDOM trial [NCT03755518]. There was a box warning.3 For my patients who are on fedratinib, I use thiamine supplementation. You could check their levels too, but that was a concern if they developed a thiamine deficiency.
DISCUSSION QUESTION
- How does the survival data of the JAK inhibitors influence your choice of therapy?
NATHAN: For the momelotinib, was there a cutoff platelet count, or irrespective of whatever the platelet count was, [was dosing the] same?
FAZAL: In the SIMPLIFY-1 trial [NCT01969838], because it was ruxolitinib vs momelotinib, the platelet count was 50,000/μL or above.4
NATHAN: I think there is an advantage with momelotinib with [transfusion independence]. Do you think eventually it will replace ruxolitinib?
FAZAL: When we assess each patient with myelofibrosis, we have different goals. If a patient is transfusion dependent or anemic, we need to talk with that patient about the goals as well…. It depends upon which patient we’re talking about. Whether it’s going to replace ruxolitinib, that’s dependent on for which patient ruxolitinib vs momelotinib is better.
REFERENCES
1. NCCN. Clinical Practice Guidelines in Oncology. Myeloproliferative neoplasms, version 1.2024. Accessed February 15, 2024. http://tinyurl.com/4jmdhff6
2. Verstovsek S, Kiladjian JJ, Vannucchi AM, et al. Early intervention in myelofibrosis and impact on outcomes: a pooled analysis of the COMFORT-I and COMFORT-II studies. Cancer. 2023;129(11):1681-1690. doi:10.1002/cncr.34707
3. Inrebic. Prescribing information. FDA. Revised August 2019. Accessed February 15, 2024. http://tinyurl.com/yjrmwbmf
4. Mesa RA, Kiladjian JJ, Catalano JV, et al. SIMPLIFY-1: a phase III randomized trial of momelotinib versus ruxolitinib in Janus kinase inhibitor-naïve patients with myelofibrosis. J Clin Oncol. 2017;35(34):3844-3850. doi:10.1200/JCO.2017.73.4418

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More