Optimal Sequencing Explored for Enzalutamide and Abiraterone in mCRPC
Within the past 5 years, several agents have become available for the treatment of metastatic castration-resistant prostate cancer (mCRPC) based on survival benefits observed in randomized clinical trials
Within the past 5 years, several agents have become available for the treatment of metastatic castration-resistant prostate cancer (mCRPC) based on survival benefits observed in randomized clinical trials. The FDA approved abiraterone acetate (Zytiga; the prodrug of abiraterone and inhibitor of CYP17A1 that blocks extragonadal, testicular, and intratumoral androgen synthesis) and enzalutamide (Xtandi; a direct inhibitor of the androgen receptor that attenuates its activation and signaling within the nucleus) in 2011 and 2012, respectively, for the treatment of mCRPC. Although each has demonstrated improved survival outcomes, the optimal sequencing of these therapies has not been established, and most patients exhibit some degree of cross-resistance.
Examining the Optimal Sequencing of Therapies
Benjamin Maughan, MD, from Johns Hopkins Sidney Kimmel Comprehensive Cancer Center in Baltimore, MD, and colleagues presented their findings on the optimal sequencing of abiraterone and enzalutamide in men with mCRPC at the Genitourinary Cancers Symposium in January 2016.1
TABLE 1
In the retrospective study of 71 patients, 58 received abiraterone followed immediately by enzalutamide (abi-to-enza) and 13 received enzalutamide followed immediately by abiraterone (enza-to-abi). The primary endpoint was combined progression-free survival (PFS), measured from initiation of the first therapy until disease progression on the second therapy. The secondary endpoint was overall survival (OS), from the start of the first therapy until death ().1
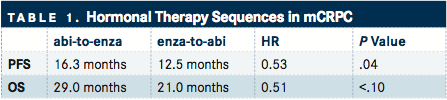
Compared with the enza-to-abi group, the abi-to-enza group had significantly longer combined PFS (16.3 vs 12.5 months; HR, 0.53; P =.04). Overall survival was also numerically longer for the abi-to-enza group, although the difference did not reach statistical significance (29.0 vs 21.0 months; HR, 0.51; P <.10). Using multivariable analyses to account for differences in prostate-specific antigen (PSA) levels, hemoglobin, visceral disease, and prior docetaxel use, the abi-to-enza sequence showed improvements in both combined PFS (HR, 2.59; P =.03) and OS (HR, 4.59; P <.01).
The results of this preliminary study suggest that the sequence of abiraterone followed by enzalutamide may produce clinically superior outcomes in men with mCRPC compared with enzalutamide followed by abiraterone. However, investigators caution that results could be due to baseline imbalances in the study population and the small sample size.
The senior author of the study, Emmanuel S. Antonarakis, MD, also with the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, noted, “The evidence that abiraterone is superior to enzalutamide in terms of optimal sequencing is very weak right now, and these data should only be viewed as hypothesis-generating. That question can only be conclusively answered by prospective studies.”
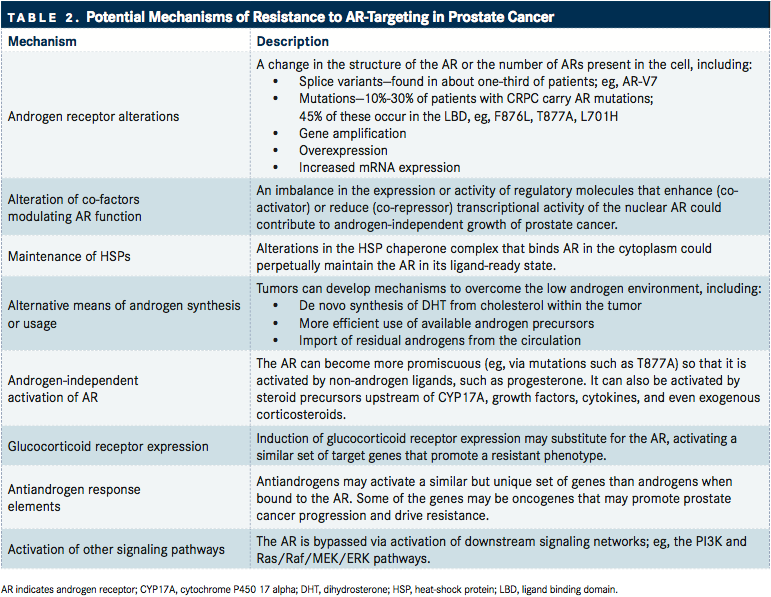
TABLE 2
To address this point, an ongoing, prospective phase II clinical trial is under way (NCT02125357). Investigators will randomize patients with mCRPC to receive either firstline abiraterone or enzalutamide, and patients will subsequently cross over to the alternative agent at the time of PSA progression. The primary endpoint will be the PSA response rate to the second-line therapy in each arm. Since androgen receptor (AR) activation drives PSA expression, PSA response will be used to determine abiraterone and enzalutamide efficacy. Secondary outcome measures will assess potential circulating biomarkers that are associated with treatment efficacy and resistance, including AR mutations as well as AR splice variants ().2
Additional studies have examined the clinical efficacy of sequential therapy and have shown modest results. In a study by Schrader et al,435 patients who had received prior docetaxel chemotherapy were treated sequentially with abiraterone followed by enzalutamide. After abiraterone treatment, 46% of patients achieved a >50% decline in PSA. Among those who were abiraterone-sensitive, 44% achieved a >50% PSA decline with enzalutamide treatment; among those who were abiraterone-insensitive, only 16% achieved a >50% PSA decline with subsequent enzalutamide.
Using data from the phase III AFFIRM clinical trial, Noonan and colleagues5 retrospectively analyzed PSA response in 30% of patients with mCRPC who had progressed with enzalutamide and were subsequently treated with abiraterone. After enzalutamide treatment (median duration, 41 weeks), 70% had a ≥30% PSA decline. However, after abiraterone treatment (median duration, 13 weeks), only 3 patients had a ≥30% PSA decline, no patients demonstrated a radiographic response, and the median time to progression was 15 weeks.5
Taken together, these studies collectively suggest that sequential therapy with either abiraterone followed by enzalutamide or vice versa is somewhat limited by the potential for cross-resistance between therapies. Although many patients will experience significant clinical benefit from abiraterone and enzalutamide given alone or in combination, 20% to 40% of patients are resistant to each therapy. Among those who respond initially, nearly all will acquire secondary resistance.68
Identifying biomarkers and examining molecular mechanisms that may be responsible for the cross-resistance between these agents is critical for determining optimal treatment sequencing and which patients are most likely to respond to a given therapy. Current therapeutic decision making requires a more nuanced approach that takes into consideration a number of patient and physician factors.
Potential Mechanisms of Resistance to Androgen Receptor‒Targeted Therapies
Several groups are examining the mechanisms of resistance to abiraterone, enzalutamide, and other next-generation ARtargeted therapies for mCRPC. According to Antonarakis, the identification of biomarkers associated with resistance requires an integrated analysis of 3 critical components of AR biology: (1) amplification or overexpression of the AR gene; (2) activating DNA mutations in the AR ligand-binding domain; and (3) AR mRNA splice variants (TABLE 2).
Androgen receptor gene amplification and overexpression are commonly observed in patients with prostate cancer in response to androgen-deprivation therapy and novel AR-targeted therapy. Ongoing studies are evaluating the extent of AR amplification and its influence on resistance to novel antiandrogen therapies. In a recent study, Arun Azad, MD, PhD, with the Vancouver Prostate Cancer Centre, in Canada, and colleagues reported on the prevalence of AR gene aberrations and their association with therapeutic resistance in CRPC in Clinical Cancer Research.9
Androgen receptor gain or amplification was identified in 28 of 62 (45%) patients. Compared with patients who had progressed on abiraterone or other agents, patients who progressed on enzalutamide had significantly higher frequency of AR amplification (53% with enzalutamide versus 17% and 21% with other agents and abiraterone, respectively; P =.02). Androgen receptor amplification was associated with inferior outcomes to both abiraterone and enzalutamide.9
Several activating mutations in the AR ligand-binding domain have also been identified. Such mutations can result in promiscuous activation by other adrenal androgens, steroidal and nonsteroidal ligands, and conversion of AR antagonists into agonists. For example, Azad et al9identified the F877L mutation in an enzalutamide-resistant patient, and the H875Y and T878A mutations in 7 abiraterone-resistant patients. The F877L mutation, which alters the conformation of the AR ligand-binding domain, has been associated with enzalutamide resistance because of its ability to drive an antagonist-to-agonist switch.10
Antonarakis and colleagues11 have examined the expression of a particular AR splice variant, AR-V7, and its associated resistance to enzalutamide and abiraterone. The results of their study were reported in The New England Journal of Medicine.
Both enzalutamide and abiraterone target the ligand-binding domain of AR, either directly or indirectly. The AR-V7 variant lacks the ligand-binding domain but retains constitutive activity as a transcription factor, potentially explaining the resistance observed with these agents.
Among enzalutamide-treated (n = 31) and abirateronetreated (n = 31) patients with mCRPC, AR-V7 was detected in circulating tumor cells of 39% and 19% of patients, respectively. Compared with patients who were AR-V7negative, AR-V7–positive patients had significantly lower PSA response rates, shorter PSA PFS, shorter clinical or radiographic PFS, and shorter OS with enzalutamide or abiraterone treatment.
Antonarakis stressed the importance of examining all 3 mechanisms of resistance associated with AR-targeted therapies. “If you only focus on one of the three pieces, you are potentially going to miss two-thirds of the explanation for resistance. To date, there has not been a single group, including our own, that has concurrently analyzed all three of those factors at the same time. That needs to be done. Only once we do that, are we truly going to uncover the majority of the resistance mechanisms to enzalutamide and abiraterone. Those types of biomarker analyses need to be built into all future trials that evaluate AR-targeted therapies or their sequencing.”
Furthermore, Antonarakis expressed that the long-term goals of these studies are to identify biomarkers that will help determine the optimal therapy sequencing based on a patient’s molecular profile, with particular attention to AR status.
“My ultimate vision is that every time a patient shifts from one CRPC therapy to another, they would receive a liquid biopsy from a blood sample, and the androgen/AR axis would be analyzed in real time. The patient would be classified based on their AR status (normal AR vs aberrant AR vs AR-null), and then a decision would be made about the next line of therapy based on their AR biology,” Antonarakis stated. “That process would be repeated every time the patient progresses on their current therapy. In that way, therapy selection would become an iterative process and would be informed at each juncture by current AR status.”
References
- Maughan BL, Suzman DL, Nadal RM, et al. Optimal sequencing of enzalutamide and abiraterone in men with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2016;34(2_suppl; abstr 308).
- ClinicalTrials.gov. Sequencing Abiraterone and Enzalutamide in mCRPC. ClinicalTrials.gov Identifier: NCT02125357.
- Maughan BL, Antonarakis ES. Androgen pathway resistance in prostate cancer and therapeutic implications. Expert Opin Pharmacother. 2015;16(10):1521-1537.
- Schrader AJ, Boegemann M, Ohlmann CH, et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur Urol. 2014;65(1):30-36.
- Noonan KL, North S, Bitting RL, et al. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol. 2013;24(7):1802-1807.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187-1197.
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005.
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138-148.
- Azad AA, Volik SV, Wyatt AW, et al. Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin Cancer Res. 2015;21(10):2315-2324.
- Joseph JD, Lu N, Qian J, et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3(9):1020-1029.
- Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028-1038.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More