Novel Targets in Relapsed and Relapsed/Refractory Multiple Myeloma
Current approaches to the management of multiple myeloma (MM), in particular the introduction of proteasome inhibitors (PIs) and immunomodulatory agents (IMiDs), have improved the survival of patients.
Noa Biran, MD

Noa Biran, MD
John Theurer Cancer Center,
Myeloma Division
Joshua Richter, MD

Joshua Richter, MD
John Theurer Cancer Center,
Myeloma Division
David S. Siegel, MD, PhD

David S. Siegel, MD, PhD
John Theurer Cancer Center,
Myeloma Division
David Vesole, MD

David Vesole, MD
John Theurer Cancer Center,
Myeloma Division
Abstract
Introduction
Current approaches to the management of multiple myeloma (MM), in particular the introduction of proteasome inhibitors (PIs) and immunomodulatory agents (IMiDs), have improved the survival of patients. However, nearly all patients ultimately relapse as drug-resistant subclones of the disease emerge. In general, treatment principles for relapsed and refractory (RR) MM depend on host factors as well as the genetics and biology of the disease. Both PI- and IMiD-based therapies are especially active in RRMM, and multidrug combinations are particularly useful in overcoming resistance in this setting. The addition of both commercially available and investigational agents to the treatment backbone of PI- and IMiD-based regimens have shown significant activity in the RR setting and are areas of current study. In particular, immune-based therapy, epigenetic modulators, and transcriptional regulators are in various stages of drug development and have shown promising activity in patients with RRMM. This review will discuss the mechanisms, clinical application, and common toxicities of both commercially available and investigational agents used in RRMM and provide a generalized and rational approach to management.Multiple myeloma (MM) is a neoplasm of plasma cells and accounts for 10% to 15% of hematologic malignancies. Current approaches to the management of newly diagnosed MM, in particular the introduction of proteasome inhibitors (PIs) and immunomodulatory agents (IMiDs), have significantly increased survival from a median of 3 to 5 years to a 5-year survival of >70% in transplant-eligible patients.1However, nearly all patients, even with the achievement of a high-quality and prolonged duration of response with initial therapy, ultimately relapse and require further therapy. In recent years, significant progress has been achieved in the characterization of MM pathophysiology in terms of gene mutations, structural chromosomal lesions, and transcription profiles.2
Tumor Heterogeneity in Multiple Myeloma
There is no standard sequence of therapy in relapsed (ie, previously treated myeloma that progresses and requires the initiation of salvage therapy) or relapsed/ refractory MM (RRMM), defined as those who, having achieved a minor response or better, relapse/progress while on salvage therapy or experience progression within 60 days of their last therapy. RRMM constitutes a specific and unmet medical need, as median overall survival (OS) ranges from 6 months to 9 months, and responses to treatment are characteristically short.3Each line of therapy results in progressively shorter durations of response, reflecting the emergence of drug resistance and development of new tumor clones.The initiation and progression of MM is influenced by mutations, translocations, and epigenetic changes, many of which have been characterized through conventional cytogenetics and fluorescent in situ hybridization (FISH). These mutations occur in different pathways and genes, which deregulate the intrinsic biology of the plasma cell or the preplasmacytic compartment (ie, clonal, preplasmacytic B cells that are not recognized by CD38/CD45 flow cytometry). Sequential acquisitions of multiple genetic events over time and through a branching, nonlinear pathway, lead to disease progression and, ultimately, treatment-resistant disease.4,5This results in a complex pathophysiology and heterogeneous genetic lesions of the tumor cells in the setting of genomic instability and molecular evolution of the disease over time and poses challenges to the efforts of improving treatment using targeted therapy. Tumor heterogeneity can be present at diagnosis, and certain tumor clones can dominate over others, which may shift over time, and influence specific classes of agents used during the course of treatment.6Subclonal heterogeneity exists in MM, as demonstrated by whole genome sequencing and next-generation sequencing approaches.7,8
Risk Stratification Determines Prognosis but Not Therapy
Although some of the genes and pathways involved in progression of disease have been elucidated, there are limitations to their applicability. In fact, recent sequencing data have demonstrated there is no single genetic change underlying this process that can be targeted therapeutically. Additionally, there is no uniform consensus on what causes these genetic events to occur. Preclinical models do not always translate in vivo, especially for MM, making it difficult to translate even the most promising investigational therapy from preclinical to clinical trials and to individualize the use of targeted agents for patients with specific molecularly defined subsets.Risk stratification in MM determines prognosis and can influence treatment strategies, although there is a lack of guidelines regarding management strategies for standard- versus high-risk patients. It is essential to define risk in patients with MM, because high-risk patients will have a median OS of 2 years or less, while standard-risk patients survive more than 10 years.9Consensus guidelines from the International Myeloma Working Group support a comprehensive cytogenetic and FISH evaluation in all patients with MM at the time of diagnosis and also at relapse.10In addition, a patient is considered to have high-risk disease if the high-risk genetic features are found at diagnosis, at relapse, or at progression. However, it is important to note that at this time, with the exception of use of bortezomib for t(4;14), there is no different treatment recommended for patients in the high-risk group compared with those with standard risk.Table 1summarizes risk classification and frequency of each genetic abnormality at diagnosis as it pertains to cytogenetics, FISH, and gene expression profiling.
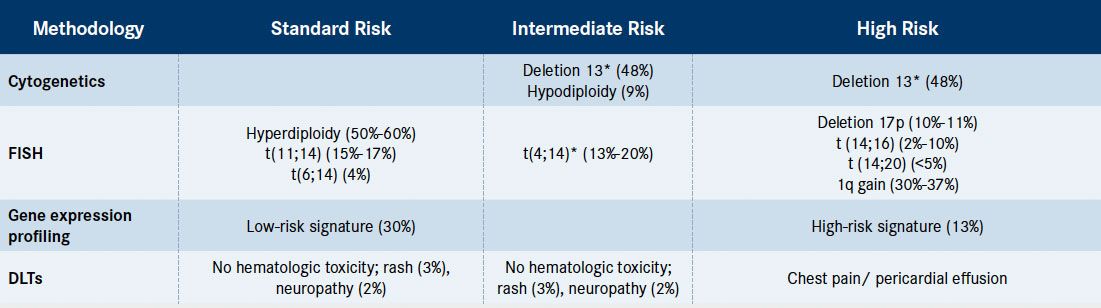
Table 1. Genetic Risk Stratification in Multiple Myeloma and Frequency of the Genetic Abnormality at Diagnosis[Mouseover or click to enlarge]
*Poor-risk abnormality can be overcome with novel therapy.
Therapeutic decisions are often dictated by practitioner- specific as well as patient-specific factors. In general, host factors, biology of disease, and genetic factors are all taken into consideration. For example, a patient with high-risk disease with adequate performance status may be treated with highly active 3- or 4-drug combinations to achieve a maximal response in the relapsed or refractory setting, although current prospective evidence does not entirely support a multidrug approach. Conversely, patients with favorable cytogenetics, indolent clinical features, and prolonged response to prior therapy may require less intensive therapy, perhaps an IMiD- or PI-based doublet. Participation in clinical trials is an important aspect of therapy in this patient population.
Approved Agents in the Treatment of Relapsed and Refractory Multiple Myeloma
Proteasome Inhibitors
Although cytogenetics and FISH allow us to begin to understand the genetics of MM, these studies are limited to a number of mutations, while MM is characterized by a wide array of molecular heterogeneity. Newer technologies such as global gene and micro-RNA expression, genome-wide DNA profiling, and next-generation sequencing technology will ultimately allow us to better investigate the genomic alterations underlying the biological and clinical heterogeneity of MM.11,12High-throughput technologies have allowed a better comprehension of the molecular basis of disease and early identification of high-risk patients. With time, we expect that these will provide more sensitive, accurate, timely, and cost-efficient methods of risk stratification.Multiple new therapeutic agents have been developed over the past decade, and the repertoire of treatment options for RRMM has expanded noticeably. The PIs bortezomib and carfilzomib, as well as the IMiDs thalidomide, lenalidomide, and pomalidomide, are now the cornerstones of almost all regimens in the RR settings. Combinations incorporating a PI and/or an IMiD in conjunction with corticosteroids and alkylating agents are often utilized.Proteasome inhibitors exert their mechanism of action through the ubiquitin-proteasome signaling pathway, which regulates both cellular homeostasis and survival and represents the predominant nonlysosomal route for degradation of misfolded, oxidized, or otherwise damaged protein.13The proteasome is a multiprotein enzyme complex, made up of two 19S caps flanking the 20S proteasome catalytic core, and degrades proteins by a conjugation of multiple ubiquitin molecules that target the substrate.
The ubiquitin-proteasome pathway activates the transcriptional factor nuclear factor-κB (NF-κB), which induces expression of growth and angiogenic factors, and promotes myelomagenesis. However, PIs have also been found to act by NF-κBindependent mechanisms: PIs induce degradation of cellcycle regulatory proteins, leading to cell apoptosis; they increase the levels of cell-cycle inhibitors, resulting in G1/S cell-cycle arrest and apoptosis and activate the JNK-signaling pathway, promoting activation of caspase-3- and caspase-8-dependent cell death; and they interfere with tumor cell–stroma cell interaction by elevation of I-κB levels, resulting in the inhibition of angiogenic cytokines and adhesion-molecule synthesis.14
Bortezomib
Bortezomib, a dipeptide with a boronic acid moiety, which binds to the 26S catalytic site of the proteasome, was the first PI to be introduced to clinical practice. It has been approved for the upfront and relapsed setting, and most recently for patients with a suboptimal response to bortezomib monotherapy in conjunction with dexamethasone.15-17
A subgroup analysis demonstrated that bortezomib is superior to dexamethasone in patients aged 65 or older and with high-risk characteristics including >1 prior therapy, International Staging System stage 2 or 3, and refractory to last prior therapy.18Furthermore, the poor prognosis associated with chromosome 13q deletion and t(4;14) is overcome with the use of bortezomib.19,20
The most common toxicities associated with bortezomib are peripheral neuropathy, gastrointestinal symptoms, fatigue, thrombocytopenia, and reactivation of herpes zoster. As such, it is essential to use concurrent antiviral prophylaxis. In the VISTA study of patients treated with melphalan and prednisone with or without intravenous (IV) bortezomib, 13% of patients in the bortezomib group experienced grade 3 peripheral neuropathy, with less than 1% having grade 4.21A pooled analysis of 2 large phase II trials (SUMMIT and CREST) was conducted to assess the frequency, characteristics, and reversibility of bortezomib- induced peripheral neuropathy. Bortezomib was held, dose-reduced, or discontinued depending on peripheral neuropathy severity. Overall, 37% of those treated with 1.3 mg/m2 and 21% of those treated with 1.1 mg/m2 had treatment-emergent peripheral neuropathy, including 9% with grade 3 or higher. Neuropathic pain and other symptoms resolved to baseline levels or improved in 71% of patients. Resolution or improvement occurred during treatment in 37% of patients and after treatment in 31% of patients. The median duration from the last dose to resolution or improvement of peripheral neuropathy was 47 days (range 1 to 529 days). Efficacy was not related to dose modifications for grade 2 or higher neuropathy.22Subcutaneous (SC) bortezomib was shown to be noninferior in efficacy to the IV route, with a significantly improved safety profile, in particular, decreased peripheral neuropathy of any grade (38% vs 53%, P = .044) and grade 3 or worse (6% vs 16%, P = .026), and it should be considered standard of care.23
A retrospective analysis of 3 different prospective studies compared 3 dosing schedules of bortezomib, melphalan, and dexamethasone (VMP). Efficacy of VMP and cumulative dose of bortezomib were similar with weekly versus twice-weekly bortezomib.24The rates of peripheral neuropathy and associated discontinuations and dose reductions were lower with VMP regimens using primarily weekly bortezomib dosing compared with twice weekly (13% grade 3 or higher peripheral neuropathy in VISTA biweekly, 14% in GIMEMA biweekly, and 7% in GIMEMA once-weekly dosing). However, the results of this study should be interpreted with caution, because the proportion of planned bortezomib dose actually delivered was significantly lower in the twiceweekly schedules compared with the once-weekly schedules, due to protocol-specified dose reductions (57% and 62.3% vs 86.1% and 90.4%, respectively). Additionally, it is difficult to interpret a betweenstudy comparison, as potential confounding factors can exist across studies. No prospective randomized study of once-weekly versus twice-weekly bortezomib dosing has been done to date.
A significant number of patients treated with bortezomib, even with once-weekly subcutaneous dosing, continue to have chronic, sometimes debilitating irreversible neuropathy.
Carfilzomib
Carfilzomib, a novel and irreversible epoxyketone PI that is selective for the chymotrypsin-like protease and binds to the β-5 subunit of the 26S proteasome, was shown in preclinical studies to induce cell apoptosis in myeloma cell lines and in primary myeloma cells from patients whose disease was resistant to therapies including bortezomib.25
In the pivotal single-arm phase II PX-171-003-A1 study, patients with a median of 5 prior lines of therapy receiving carfilzomib alone, 80% of whom were refractory to or intolerant of bortezomib and lenalidomide, achieved a 23% overall response rate (ORR)defined as partial response (PR) or better.26Median duration of response (DOR) and OS were 7.8 and 15.6 months, respectively. Carfilzomib was given as 20 mg/m2 IV twice weekly for 3 of 4 weeks in cycle 1 and 27 mg/m2 for up to 12 cycles thereafter with low-dose dexamethasone as premedication for the first 2 cycles. As a single agent, carfilzomib was not able to overcome the impact of high-risk cytogenetics.27
Common adverse events (AEs) associated with carfilzomib included anemia, fatigue, nausea, and thrombocytopenia. The incidence of peripheral neuropathy was much less than that observed with bortezomib, present in 12% any grade and only 1% grade 3 or greater. Dyspnea was seen in 34% of patients but thought to be related to carfilzomib in 17% and grade 3 or greater in 3.4%.26 The safety profile has been confirmed by data from 4 phase II studies including 526 RRMM patients treated with carfilzomib.28-30
Carfilzomib is safe in patients with varying degrees of renal impairment, including those on chronic hemodialysis, which does not influence pharmacokinetics of carfilzomib. In addition, renal impairment did not affect response or AEs associated with carfilzomib.30 In the phase III ASPIRE trial of lenalidomide and dexamethasone with or without carfilzomib, dyspnea of any grade was seen in 19.4% versus 14.9% of the carfilzomib and control group, and grade 3 or higher in 2.8% versus 1.8%, respectively.31Cardiac failure of any grade was seen in 6.4% of the carfilzomib group versus 4.1% of the control group. Recent studies have shown that doses of carfilzomib that are twice the amount of those approved (up to 70 mg/m2 once weekly) are generally well tolerated when infused over 30 minutes.32
Investigational Proteasome Inhibitors
Immunomodulatory Agents
Several PIs, including ixazomib, marizomib, and oprozomib, are under investigation in phase I/II clinical studies. The investigational PI with which there is the most experience is ixazomib (MLN9708), a second-generation boronate PI, the first oral PI to be evaluated in MM clinical trials. Unlike bortezomib, at higher concentrations, it can inhibit the caspaselike proteolytic β-5 site of the 20S proteasome and trypsin-like β-2 proteolytic sites.33A phase II study evaluated ixazomib administered as a single agent in patients with RRMM who were bortezomib naïve. With a median follow-up of 7 months, OS at 6 months was 96%. Common grade 3 or 4 AEs included diarrhea, thrombocytopenia, nausea, and fatigue and occurred in 56% of patients.34In a preliminary analysis from a phase Ib/II study of oprozomib and dexamethasone in 29 patients with RRMM and a median of 3 prior lines of therapy, best ORR was 41.7%, and most common toxicities included diarrhea (83%), nausea (79%), and vomiting (62%), in addition to anemia and pneumonia.35The mechanism of IMiDs has not been completely elucidated. Following more than a decade of research on the mechanism of thalidomide, the first IMiD to be introduced into the MM armamentarium, preclinical studies identified E3 ligase protein cereblon as a direct molecular target for the teratogenicity of thalidomide, binding directly to thalidomide analog affinity beads. Cereblon, of which its auto-ubiquitination was inhibited by thalidomide, is highly conserved from plants to mammals, and the mRNA for human, rat, and mouse cereblon is ubiquitously expressed.36Cereblon has recently been proposed as a mediator of the anti-MM activity of lenalidomide and pomalidomide as well37,38and is a substrate receptor of Cul4- E3 ubiquitin ligase complex, and thus recognizes proteins destined for degradation. Binding of cereblon and IMiDs inhibits or modifies the specificity of the entire ubiquitin proteasome complex, which partly explains their antitumor effects. In addition, a correlation between cereblon gene expression and effectiveness of treatment in MM patients treated with IMiDs was confirmed.39
Follow-up studies have suggested that IMiDs are not simply cereblon antagonists but, instead, alter the substrate specificity of cereblon to include proteins important in myeloma. Numerous novel cereblon-binding proteins altered in MM cells after IMiD treatment have been implicated, particularly the Ikaros transcription factor zinc-finger proteins 1 and 3, IKZF1 and IKZF3, which were previously demonstrated to regulate B-cell activation and differentiation. Recently, 2 landmark studies both independently established that IKZF1 and IKZF3 are substrates of cereblon ubiquitin ligase.40,41Analysis of myeloma cell lines have linked lenalidomide’s antimyeloma activity to down-regulation of IKZF1 and IKZF3, and conversely, expression of the stabilized versions of IKZF1 or IKZF3 conferred lenalidomide resistance to myeloma cells.42Another study found that low IKZF1 expression levels were associated with a poor response to IMiDs and shorter OS.42This observation may be explained by the fact that myeloma cells surviving with low IKZF1 are less dependent on IKZF1-associated signaling for proliferation and survival and are therefore resistant to IMiDs. Thus, novel cereblon-binding proteins altered in MM cells after IMiD treatment such as IKZF1 and IKZF3 may serve as biomarkers for clinical outcomes.
Thalidomide
In the initial phase II study of single-agent thalidomide, 84 individuals with RRMM received thalidomide at doses ranging from 200 to 800 mg daily. The ORR was 32%, with an OS rate of 48%.43Importantly, in RRMM, thalidomide plus dexamethasone has not been compared prospectively with high-dose dexamethasone alone. However, in a study comparing thalidomide and dexamethasone to dexamethasone alone in 470 patients with newly diagnosed MM, common AEs included constipation (49.6% vs 21.1%), peripheral edema (34.2% vs 24.6%), tremor (26.5% vs 12.5%), fatigue (21.4% vs 15.5%), and deep vein thrombosis (11.5% vs 1.7%).44Unlike the peripheral neuropathy seen with bortezomib, the peripheral neuropathy associated with thalidomide is caused by loss of large-diameter myelinated nerve fibers and axonal injury and is directly related to cumulative exposure.45
Lenalidomide
Lenalidomide is a structural analog of thalidomide and was developed in the search for a drug with more potency and less toxicity. Two large multicenter phase III studies including a total of 704 RRMM patients, MM-009 and MM-010, demonstrated the activity of lenalidomide plus dexamethasone (Ld).46,47Compared with placebo/dexamethasone, Ld induced a significantly higher ORR (60% vs 24%; P <.001), complete response (CR) (16% vs 3.4%; P <.001), and OS (HR 0.66; P = .03). A subset analysis, however, demonstrated a significantly greater median OS (not reached vs 30.8 months; P = .028) and progression-free survival (PFS) (14 vs 9.5 months; P = .047) in patients who were in their first relapse compared with patients who had >1 line of prior therapy.48This may confirm the notion that MM becomes more heterogeneous with each line of therapy and that due to various subclones and resistant disease, more intense therapy is required as the disease progresses.
Significant toxicities associated with Ld include myelosuppression and venous thromboembolic events. Thromboprophylaxis is required when using an IMiD and a corticosteroid.
Pomalidomide
Pomalidomide is also a structural analog of thalidomide and was developed before lenalidomide. In the pivotal randomized phase II study of pomalidomide with or without dexamethasone, MM-002, patients with RRMM who were resistant to both IMiDs and PIs (including both bortezomib and carfilzomib) received either Pd (pomalidomide 4 mg daily days 1-21 in a 28-day cycle with dexamethasone) or pomalidomide alone.49ORR was 34% in those who received pomalidomide and dexamethasone, and median PFS was significantly longer for the combination (4.2 vs 2.7 months, P = .003). Median DOR was 8.3 versus 10.7 months in patients who received pomalidomide with or without dexamethasone, respectively. More importantly, activity was shown in patients with high-risk features including del 17p, t(4;14) and in those with extramedullary disease.
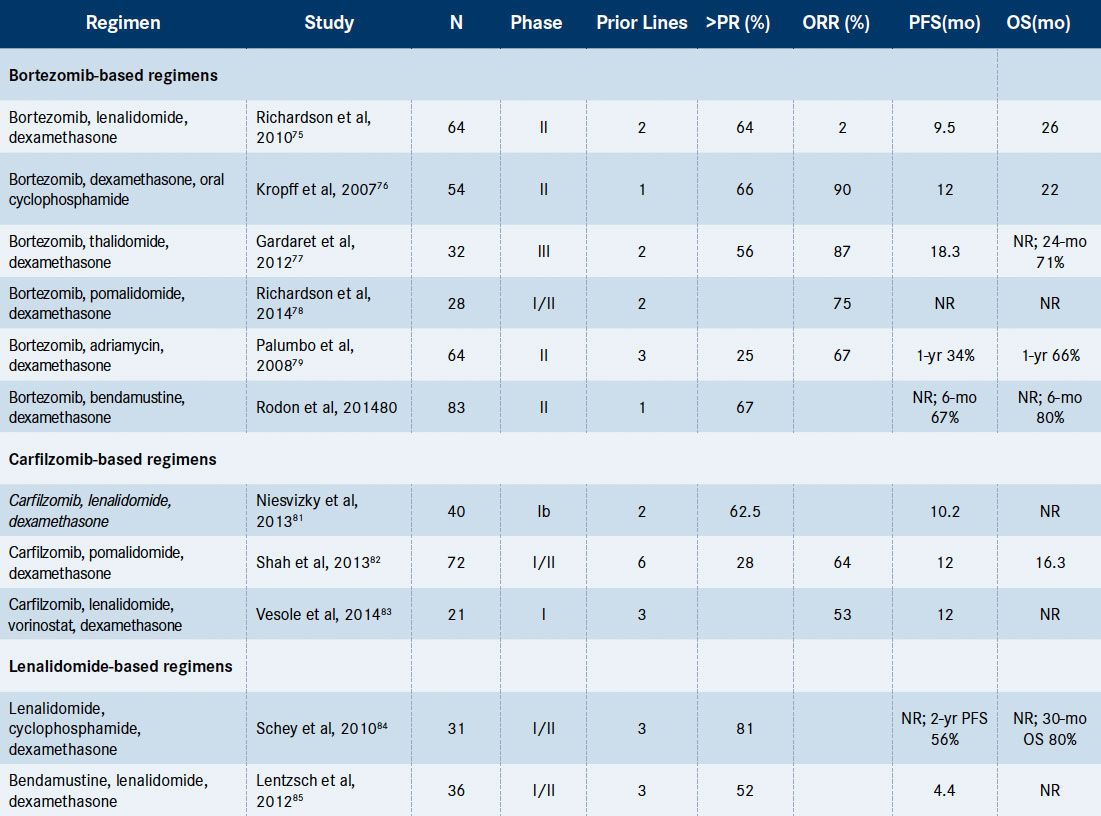
Table 2. Selected Multi-Drug Combination Regimens in the Relapsed/Refractory Setting[Mouseover or click to enlarge]
Abbreviations: NR = not reached; ORR = overall response rate (partial response or better); OS = overall survival; PFS = progression-free survival.
Pomalidomide plus low-dose dexamethasone was compared with high-dose dexamethasone in a 2:1 randomized phase III study involving 302 patients with RRMM and demonstrated the superiority of the combination in terms of both PFS (4 vs 1.9 months, P <.0001) and OS (12.7 vs 8.1 months, P = .028), including in patients with moderate- to high-risk cytogenetics.50
Favorable tolerability was observed. Common AEs included myelosuppression, with minimal peripheral neuropathy (1%) and venous thromboembolic events (1%). Discontinuation due to AEs occurred in 9% of patients; the main reasons for therapy discontinuation were infections (34%), pneumonia (14%), thrombocytopenia (22%), and febrile neutropenia (48%).
Selected Investigational Agents Currently in Clinical Trials
Table 2highlights selected multidrug combination regimens that have shown significant activity in RRMM.Translating promising investigational therapies from the bench to the bedside has presented a challenge in the treatment of RRMM. Several recent advances in the understanding of the pathogenesis of MM have led to promising therapeutic agents and combinations.
Epigenetic Modulators
Epigenetic changes have been shown to play a role in MM pathogenesis, as several genes for histonemodifying enzymes (such as MMSET, MLL, and MLL2) have been found to be recurrently mutated in MM cells.2,6Through increased acetylation of intracellular protein, alterations occur in transcription and activity of tumor suppressor genes. Deacetylase inhibition has demonstrated preclinical synergy with bortezomib in MM cells.51As such, pan-histone deacetylase inhibitors such as vorinostat, panobinostat, and romidepsin, as well as the HDAC6-specific inhibitor ACY-1215, have demonstrated significant anti-MM activity in preclinical studies and have been evaluated in the relapsed and refractory setting in various combinations.
In the phase I multicenter, open-label, doseescalating study of vorinostat in combination with lenalidomide and dexamethasone in patients with relapsed MM or RRMM, a confirmed PR or better was reported in 14 of 30 (47%) patients, including 31% of patients previously treated with lenalidomide and 32% of patients previously treated with PIs.52This suggests that vorinostat in combination with lenalidomide and dexamethasone has the potential to recapture response in patients who are lenalidomide- and bortezomib-refractory. The most common AEs were anemia (58%), thrombocytopenia (58%), diarrhea (55%), fatigue (55%), and cough (45%). Side effects were generally manageable with dose reduction and supportive care. Vorinostat, although not approved for treatment of MM, is included in the National Comprehensive Cancer Network (NCCN) guidelines.53
In the phase II study PANORAMA 2, of panobinostat 20 mg orally 3 times weekly, bortezomib 1.3 mg/m2 twice weekly, and dexamethasone 20 mg on days of and after bortezomib, 34.5% of heavily pretreated patients who were bortezomib-refractory achieved a PR or better.54The phase III trial PANORAMA 1 found panobinostat, bortezomib, and dexamethasone to significantly improve PFS compared with placebo, bortezomib, and dexamethasone, with a median of 12 versus 8.1 months (P <.0001) in 387 patients who received 1 to 3 prior therapies and were not bortezomib-refractory.55Common grade 3 or greater toxicities included thrombocytopenia (67% vs 31%), neutropenia (35% vs 11%), and diarrhea (26% vs 8%). On-treatment deaths occurred in 8% and 5%, respectively.
Immune-Based Therapies
Immune-based therapies have shown both preclinical and clinical activity in patients with RRMM. Use of monoclonal antibodies against specific surface molecules in MM cells have shown promising activity.
Elotuzumab, which is directed against CS-1, a glycoprotein that is highly specific to plasma cells (also expressed in natural killer cells and CD8+ T cells), is the best evaluated agent of the monoclonal antibodies. Enrollment for a phase III trial comparing lenalidomide and dexamethasone with and without elotuzumab has been completed. The combination of elotuzumab with lenalidomide and dexamethasone has shown an ORR >80% in relapsed patients who had a median of 3 prior lines of therapy and a prolonged PFS of 33 months in the most recent update.56
CD38 and CD138 are other antigens of the plasma cells that have been targeted by monoclonal antibodies. Daratumumab is an anti-CD38 antibody designed to induce the killing of myeloma cells by direct cytotoxicity and by enrichment of the immune function through antigen-dependent cellular cytotoxicity. Heavily pretreated patients treated with daratumumab alone achieved a 42% ORR.57,58Another anti-CD38 monoclonal antibody, SAR650984, with a similar profile, is already being tested in phase I clinical trials.59Indatuximab ravtansine (BT062) in combination with lenalidomide and low-dose dexamethasone is currently being evaluated in patients with relapsed MM and RRMM. Preliminary results in 15 heavily pretreated patients (median 4 lines of prior therapy) reveal an ORR of 78%, including 1 patient with a CR. The most common AEs were fatigue, hypokalemia, and diarrhea.60
Another class of immune regulatory cells, myeloid- derived suppressor cells, which represent a diverse group of immature myeloid cells capable of suppressing immune responses, are currently being harnessed as a therapeutic strategy, as they have been found to be increased in the peripheral blood and bone marrow aspirate of patients with MM.61,62
Immune checkpoint signaling plays an important role in providing the tumor-promoting, immune-suppressive microenvironment in MM. Blockade of PD-1/PD-L1 signaling has been shown to induce anti-MM immune responses that can be enhanced by lenalidomide. Statistical analysis of Intergroupe Francophone du Myelome MM data demonstrated that the majority of patient MM cells have increased PD-L1 mRNA compared with healthy donors.63Clinical trials of PD-1, PD-L1, and other immune checkpoint inhibitors in RRMM are under way. In addition, combination vaccines consisting of dendritic cells in combination with inhibition of the T-cell down-regulator PD-1 have shown to promote T-cell tolerance after autologous stem cell transplantation (ASCT).64
Chimeric antigen receptor (CAR) T cells are cellular immunotherapy in early development for treatment of MM; they are synthetic transmembrane proteins designed to confer an alternative specificity upon immune effector cells, typically Tlymphocytes. An extracellular domain recognizes a cell-surface antigen specific for the target, and an intracellular domain initiates signal transduction necessary for T-cell activation upon antigen binding. T-cell activation requires not only recognition of a target epitope by the T-cell receptor but also accessory signals delivered by antigenpresenting cells.65Selection of the appropriate CAR target is of utmost importance, as targets should be functionally essential for the oncologic phenotype of the target cell to minimize the chance of resistance through target down-regulation. Ongoing studies of CAR T cells in MM include one using a CAR-targeting kappa immunoglobulin light chain (NCT00881920), one targeting anti-CD138 (NCT01886976), and one targeting B-cell maturation antibody (NCT02215967).
Transcriptional Regulators
Regulators of transcription have served as targets of investigational therapy in MM. Selinexor (KPT-330) is a potent, selective inhibitor of a nuclear transport protein overexpressed in MM and identified as an essential protein for MM growth. Early clinical trials have shown promising activity of KPT-330 in heavily pretreated patients. The most common drug-related AEs included myelosuppression, impaired renal function, and gastrointestinal toxicity.66
Another agent, ARRY-520, which works by inhibiting the kinesin spindle protein (KSP), has shown preclinical activity in MM by degradation of MCL-1. In the clinical setting, early results appear promising both as a single agent and as part of combination therapy.67,68Interim results from a dose-escalation study of ARRY-520 in combination with bortezomib and dexamethasone in patients with RRMM, all PI-refractory with a median of 5 prior regimens, exhibited preliminary signs of efficacy. The most commonly reported AEs include anemia, diarrhea, pyrexia, and upper respiratory tract infection.69
Clinical Pearls
- Multiple myeloma is a heterogeneous disease for which risk stratification can help determine prognosis but does not dictate treatment by current guidelines.
- Two novel agents recently approved for the treatment of relapsed and relapsed/refractory multiple myeloma include carfilzomib, a proteasome inhibitor, and pomalidomide, an immunomodulatory agent.
- Immune-based therapy, epigenetic modulators, and transcriptional regulators are in various stages of drug development and have shown promising activity in patients with relapsed and relapsed/refractory multiple myeloma.
Discussion
Patients with MM and a p53 (or 17p) deletion have been shown to have inferior outcomes, and there is no agent to date that has shown to consistently improve or overcome these poor prognostic findings. However, restoration of p53 function in cells with deletion of both alleles has demonstrated in vitro and in vivo activity. MDM2 inhibition, for example, through the small molecule APR-246, is capable of restoring transcriptional activity of unfolded wildtype or mutant p53 and is presently being evaluated in phase I clinical trials.70The management of relapsed MM and RRMM requires a systematic approach based on the characteristics of both the host and the underlying biology of the disease. Currently, there are several tools, such as cytogenetics, FISH, and gene expression profiling that can help us scratch the surface of the disease biology and lead to more informed decisions regarding therapy.
The introduction of PI- and IMiD-based therapies has changed the natural course of the disease for many patients. However, the clinical outcomes remain poor when patients have high-risk disease, experience relapse, or become refractory to these standard treatments. Other high-risk populations presenting challenges that are beyond the scope of this article include those with renal insufficiency, frail patients, and the elderly. As demonstrated inTable 2, numerous multidrug combinations have shown activity and significant efficacy in patients with RRMM. The question is, when do you use each combination and in what sequence? It is also unknown whether sequential therapy versus the use of multidrug combinations in the relapsed setting can impact OS. Evidence at this time is insufficient to support a unique sequence of therapies, and the evidence for effectiveness of retreatment versus switching therapy to another class of drugs is limited.
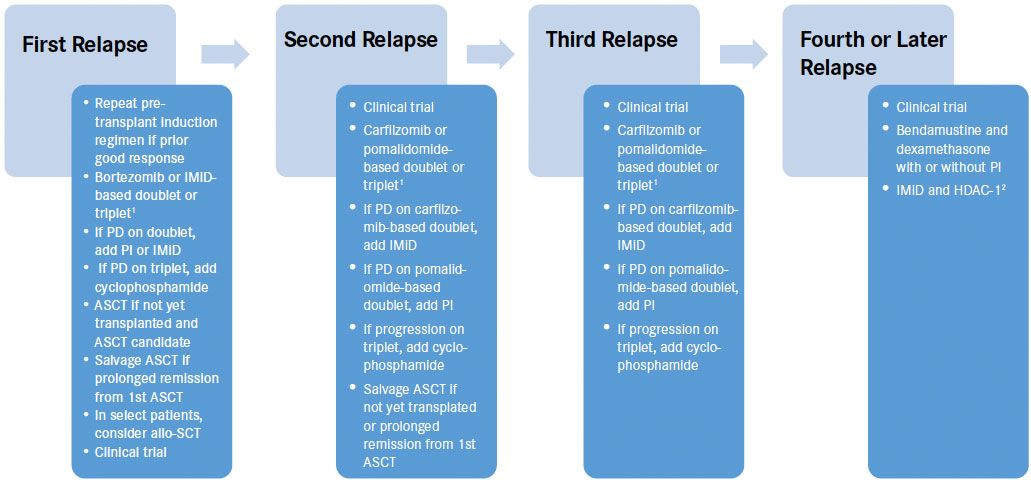
Figure 1. Generalized management approach to relapse multiple myeloma[Mouseover or click to enlarge]
ASCT indicates autologous stem cell transplant; HDAC-1, histone deacetylase inhibitor; IMiD, immunomodulatory agent; PD, progression of disease; PI, proteasome inhibitor;
1Use triplet if aggressive relapse, defined by relapse within 1 year, extramedullary plasmacytoma, plasma cell leukemia, or high-risk cytogenetics, FISH or GEP
2consider vorinostat
Figure 1demonstrates a generalized rational approach to the management of relapsed MM. At first relapse, it is reasonable to retreat with the same regimen used for induction, as long as the prior response was adequate. It may be prudent to repeat high-dose chemotherapy with autologous stem cell rescue if the patient has had a prolonged remission after high-dose chemotherapy with ASCT. For the second and third relapse, carfilzomib- or pomalidomide- based doublets or triplets may be used. The addition of cyclophosphamide to any triplet regimen may provide for additional response upon progression. Refractoriness to one agent does not necessarily indicate refractoriness to the same agent in combination with other agents or with the addition of a third or fourth drug. At any relapse, a salvage ASCT or clinical trial should be considered. In fact, there is benefit to participation in clinical trials early in the disease course when performance status is good and in the absence of end-organ compromise, rather than at later lines of therapy when performance status has declined and pancytopenia or organ dysfunction may limit clinical trial eligibility.
In practice, several factors are taken into account when making treatment decisions for an individual patient. The prior treatment including depth and quality of response, patient comorbidities, treatment- related toxicities, and patient lifestyle (eg, oral vs infusion-based therapy; once- vs twice-weekly regimen in a patient currently working) must all be considered.
References
- Kumar SK, Rajkumar SV. The current status of minimal residual disease assessment in myeloma. 2014;28(2):239-240.
- Chapman MA, Lawrence MS, Keats JJ, et al. Initial genome sequencing and analysis of multiple myeloma. 2011;471(7339):467-472.
- Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. 2012;26(1):149- 157.
- Brioli A, Melchor L, Walker BA, Davies FE, Morgan GJ. Biology and treatment of myeloma. 2014;14S:S65-S70.
- Keats JJ, Chesi M, Egan JB, et al. Clonal competition with alternating dominance in multiple myeloma. 2012;120(5):1067-1076.
- Egan JB, Shi CX, Tembe W, et al. Whole-genome sequencing of multiple myeloma from diagnosis to plasma cell leukemia reveals genomic initiating events, evolution, and clonal tides. 2012;120(5):1060-1066.
- Bolli N, Avet-Loiseau H, Wedge DC, et al. Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. 2014;5:2997.
- Waheed S, Shaughnessy JD, van Rhee F, et al. International staging system and metaphase cytogenetic abnormalities in the era of gene expression profiling data in multiple myeloma treated with total therapy 2 and 3 protocols. 2011;117(5):1001-1009.
- Chng WJ, Dispenzieri A, Chim CS, Fonseca R, Goldschmidt H, Lentzsch S, et al. IMWG consensus on risk stratification in multiple myeloma. 2014;28(2):269-277.
- Munshi NC, Anderson KC, Bergsagel PL, et al. Consensus recommendations for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2. 2011;117(18):4696-4700.
- Egan JB, Kortuem KM, Kurdoglu A, et al. Extramedullary myeloma whole genome sequencing reveals novel mutations in Cereblon, proteasome subunit G2 and the glucocorticoid receptor in multi drug resistant disease. 2013;161(5):748-751.
- Adamia S, Reiman T, Crainie M, et al. Intronic splicing of hyaluronan synthase 1 (HAS1): a biologically relevant indicator of poor outcome in multiple myeloma. 2005;105(12):4836-4844.
- Ciechanover A. The ubiquitin-proteasome proteolytic pathway. 1994;79(1):13-21.
- Ruggeri B, Miknyoczki S, Dorsey B, Hui AM. The development and pharmacology of proteasome inhibitors for the management and treatment of cancer. 2009;57:91-135.
- Richardson PG, Sonneveld P, Schuster MW, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. 2005;352(24):2487- 2498.
- Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. 2007;110(10):3557-3560.
- Jagannath S, Richardson PG, Barlogie B, et al. Bortezomib in combination with dexamethasone for the treatment of patients with relapsed and/ or refractory multiple myeloma with less than optimal response to bortezomib alone. 2006;91(7):929-934.
- Richardson PG, Sonneveld P, Schuster MW, et al. Safety and efficacy of bortezomib in high-risk and elderly patients with relapsed multiple myeloma. 2007;137(5):429-435.
- Jagannath S, Richardson PG, Sonneveld P, et al. Bortezomib appears to overcome the poor prognosis conferred by chromosome 13 deletion in phase 2 and 3 trials. 2007;21(1):151-157.
- Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. 2010;376(9758):2075-2085.
- Spicka I, Mateos MV, Redman K, Dimopoulos MA, Richardson PG. An overview of the VISTA trial: newly diagnosed, untreated patients with multiple myeloma ineligible for stem cell transplantation. 2011;3(9):1033- 1040.
- Richardson PG, Sonneveld P, Schuster MW, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. 2009;144(6):895-903.
- Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. 2011;12(5):431-440.
- Mateos MV, Bringhen S, Richardson PG, et al. Bortezomib cumulative dose, efficacy, and tolerability with three different bortezomibmelphalan- prednisone regimens in previously untreated myeloma patients ineligible for high-dose therapy. 2014;99(6):1114-1122.
- Kuhn DJ, Chen Q, Voorhees PM, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. 2007;110(9):3281-3290.
- Siegel DS, Martin T, Wang M, et al. A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. 2012;120(14):2817-2825.
- Jakubowiak AJ, Siegel DS, Martin T, et al. Treatment outcomes in patients with relapsed and refractory multiple myeloma and high-risk cytogenetics receiving single-agent carfilzomib in the PX-171-003-A1 study. 2013;27(12):2351-2356.
- Vij R, Wang M, Kaufman JL, et al. An open-label, single-arm, phase 2 (PX- 171-004) study of single-agent carfilzomib in bortezomib-naive patients with relapsed and/or refractory multiple myeloma. 2012;119(24):5661- 5670.
- Jagannath S, Vij R, Stewart AK, et al. An open-label single-arm pilot phase II study (PX-171-003-A0) of low-dose, single-agent carfilzomib in patients with relapsed and refractory multiple myeloma. 2012;12(5):310-318.
- Badros AZ, Vij R, Martin T, et al. Carfilzomib in multiple myeloma patients with renal impairment: pharmacokinetics and safety.2013;27(8):1707-1714.
- Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. 2015;372(2):142-152.
- Palumbo A, Rossi D, Bringhen S, et al. Weekly carfilzomib, cyclophosphamide, and dexamethasone (wCCd) in newly diagnosed multiple myeloma patients: a phase I-II study. Presented at:American Society of Hematology Annual Meeting and Exposition. December 7, 2014; San Francisco, California. Abstract 19442014;(653):abstract 175.
- Chauhan D, Tian Z, Zhou B, et al. In vitro and in vivo selective antitumor activity of a novel orally bioavailable proteasome inhibitor MLN9708 against multiple myeloma cells. 2011;17(16):5311-5321.
- Kumar SK, Roy V, Reeder C, et al. Phase 2 trial of single agent MLN9708 in patients with relapsed multiple myeloma not refractory to bortezomib. Presented at:American Society of Hematology Annual Meeting and Exposition. December 7, 2013; New Orleans, Louisiana. Abstract 1944.
- Hari PN, Shain KH, Voorhees PM, et al. Oprozomib and dexamethasone in patients with relapsed and/or refractory multiple myeloma: initial results from the dose escalation portion of a phase 1b/2, multicenter, open-label study. Presented at:American Society of Hematology Annual Meeting and Exposition. December 7, 2014; San Francisco, California. Abstract 3453.
- Higgins JJ, Tal AL, Sun X, et al. Temporal and spatial mouse brain expression of cereblon, an ionic channel regulator involved in human intelligence. 2010;24(1):18-26.
- Stankova M, Besse L, Sedlarikova L, Vrabel D, Hajek R, Sevcikova S. [Cereblon - a new target of therapy in the treatment of multiple myeloma]. 2014;27(5):326-330.
- Zhu YX, Braggio E, Shi CX, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. 2011;118(18):4771-4779.
- Lopez-Girona A, Mendy D, Ito T, et al. Cereblon is a direct protein target for immunomodulatory and antiproliferative activities of lenalidomide and pomalidomide. 2012;26(11):2326-2335.
- Kronke J, Udeshi ND, Narla A, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. 2014;343(6168):301-305.
- Lu G, Middleton RE, Sun H, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. 2014;343(6168):305-309.
- Zhu YX, Braggio E, Shi CX, et al. Identification of cereblon-binding proteins and relationship with response and survival after IMiDs in multiple myeloma. 2014;124(4):536-545.
- Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. 1999;341(21):1565-1571.
- Rajkumar SV, Rosinol L, Hussein M, et al. Multicenter, randomized, double-blind, placebo-controlled study of thalidomide plus dexamethasone compared with dexamethasone as initial therapy for newly diagnosed multiple myeloma. 2008;26(13):2171-2177.
- Grover JK, Uppal G, Raina V. The adverse effects of thalidomide in relapsed and refractory patients of multiple myeloma. 2002;13(10):1636- 1640.
- Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. 2007;357(21):2133- 2142.
- Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. 2007;357(21):2123-2132.
- Stadtmauer EA, Weber DM, Niesvizky R, et al. Lenalidomide in combination with dexamethasone at first relapse in comparison with its use as later salvage therapy in relapsed or refractory multiple myeloma. 2009;82(6):426-432.
- Richardson PG, Siegel DS, Vij R, et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. 2014;123(12):1826-1832.
- San Miguel J, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. 2013;14(11):1055-1066.
- Carew JS, Giles FJ, Nawrocki ST. Histone deacetylase inhibitors: mechanisms of cell death and promise in combination cancer therapy. 2008;269(1):7-17.
- Siegel DS, Richardson P, Dimopoulos M, et al. Vorinostat in combination with lenalidomide and dexamethasone in patients with relapsed or refractory multiple myeloma. 2014;4:e202.
- National Comprehensive Cancer Network. NCCN guidelines®: multiple myeloma. http://www.nccn.org/professionals/physician_gls/f_ guidelines.asp#myeloma. Accessed January 23, 2015.
- Richardson PG, Schlossman RL, Alsina M, et al. PANORAMA 2: panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory myeloma. 2013;122(14):2331-2337.
- Richardson PG, Hungria VT, Yoon SS, et al. PANORAMA 1: a randomized, double blind, phase 3 study of panobinostat or placebo plus bortezomib and dexamethasone in relapsed or relapsed and refractory multiple myeloma. 2014;32(suppl). Abstract 8510.
- Lonial S, Vij R, Harousseau JL, et al. Elotuzumab in combination with lenalidomide and low-dose dexamethasone in relapsed or refractory multiple myeloma. 2012;30(16):1953-1959.
- Plesner T, Lokhorst H, Gimsing P, Nahi H, Lisby S, Richardson PG. Daratumumab, a CD38 monoclonal antibody in patients with multiple myeloma - data from a dose-escalation phase I/II study. Presented at:American Society of Hematology Annual Meeting and Exposition. December 9, 2012; Atlanta, Georgia. Abstract 73.
- Lokhorst HM, Plesner T, Gimsing P, et al. Phase I/II dose escalation study of daratumumab in patients with relapsed or refractory multiple myeloma. 2013;31(15 suppl):8512.
- Martin TG, Strickland SA, Glenn M, Zheng W, Daskalakis N, Mikhael JR. SAR650984, a CD38 monoclonal antibody in patients wtih selected CD38+ hematological malignancies - data from a dose-escalation phase I study. Presented at:American Society of Hematology Annual Meeting and Exposition. December 6, 2013; New Orleans, Louisiana. Abstract 284.
- Kelly KR, Chanan-Khan A, Somlo G, et al. Indatuximab ravtansine (BT062) in combination with lenalidomie and low-dose dexamethasone in patients with relapsed and/or refractory multiple myeloma: clinical activity in len/ dex refractory patients. 2013;758(653):393-394.
- Van Valckenborgh E, Schouppe E, Movahedi K, et al. Multiple myeloma induces the immunosuppressive capacity of distinct myeloid-derived suppressor cell subpopulations in the bone marrow. 2012;26(11):2424- 2428.
- Brimnes MK, Vangsted AJ, Knudsen LM, et al. Increased level of both CD4+FOXP3+ regulatory T cells and CD14+HLA-DR(-)/low myeloidderived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. 2010;72(6):540-547.
- Gorgun GT, Cowens K, Paula S, et al. Targeting immune suppressive microenvironment by immune checkpoint blockade in multiple myeloma. Presented at:American Society of Hematology Annual Meeting and Exposition. December 6, 2014; San Francisco, California. Abstract 27.
- Rosenblatt J, Glotzbecker B, Mills H, et al. PD-1 blockade by CT-011, anti- PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. 2011;34(5):409-418.
- Garfall AL, Fraietta JA, Maus MV. Immunotherapy with chimeric antigen receptors for multiple myeloma. 2014;17(91):37-46.
- Chen CI, Gutierrez M, de Nully Brown P, et al. Anti tumor activity of selinexor (KPT-330), a first in class oral selective inhibitor of nuclear export (XP01/CRM1) antagonist in patients with relapsed/refractory multiple myeloma or Waldenstroms macroglobulinemia. 2013;(653)1942.
- Tunquist BJ, Woessner RD, Walker DH. Mcl-1 stability determines mitotic cell fate of human multiple myeloma tumor cells treated with the kinesin spindle protein inhibitor ARRY-520. 2010;9(7):2046-2056.
- Shah JJ, Feng L, Thomas SK, et al. Phase I study of the novel kinesin spindle protein inhibitor ARRY-520 and carfilzomib in patients with relapsed and/or refractory multiple myeloma. 2013;122(21).
- Chari A, Htut M, Zonder J, et al. A phase 1 study of ARRY-520 with bortezomib and dexamethasone in relapsed or refractory multiple myeloma. Presented at: American Society of Hematology Annual Meeting and Exposition. December 7, 2013; New Orleans, LA. Abstract 1938.
- Lehmann S, Bykov VJ, Ali D, et al. Targeting p53 in vivo: a first-in-human study with p53-targeting compound APR-246 in refractory hematologic malignancies and prostate cancer. 2012;30(29):3633-3639.
- Avet-Loiseau H, Attal M, Moreau P, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. 2007;109(8):3489-3495.
- Bergsagel PL, Kuehl WM. Chromosome translocations in multiple myeloma. 2001;20(40):5611-5622.
- Nahi H, Sutlu T, Jansson M, Alici E, Gahrton G. Clinical impact of chromosomal aberrations in multiple myeloma. 2011;269(2):137-147.
- Shaughnessy JD Jr, Zhan F, Burington BE, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. 2007;109(6):2276-2284.
- Richardson P, Xie W, Jakubowiak AJ, et al. Phase II trial of lenalidomide, bortezomib and dexamethasone in patients with relapsed and relapsed/ refractory multiple myeloma: updated efficacy and safety data after >2 years of follow-up. 2010;1(16:3049).
- Kropff M, Bisping G, Schuck E, et al. Bortezomib in combination with intermediate-dose dexamethasone and continuous low-dose oral cyclophosphamide for relapsed multiple myeloma. 2007;138(3):330-337.
- Garderet L, Iacobelli S, Moreau P, et al. Superiority of the triple combination of bortezomib-thalidomide-dexamethasone over the dual combination of thalidomide-dexamethasone in patients with multiple myeloma progressing or relapsing after autologous transplantation: the MMVAR/IFM 2005-04 Randomized Phase III Trial from the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. 2012;30(20):2475-2482.
- Richardson PG, Siegel DS, Vij R, et al. Randomized, open label phase 1/2 study of pomalidomide (POM) alone or in combination with low-dose dexamethasone (LoDex) in patients (Pts) with relapsed and refractory multiple myeloma who have received prior treatment that includes lenalidomide (LEN) and bortezomib (BORT): phase 2 results. Presented at: American Society of Hematology Annual Meeting and Exposition. December 12, 2011; San Diego, California.
- Palumbo A, Gay F, Bringhen S, et al. Bortezomib, doxorubicin and dexamethasone in advanced multiple myeloma. 2008;19(6):1160-1165.
- Rodon P, Hulin C, Pegourie B, et al. Phase 2 study of bendamustine, bortezomib and dexamethasone as second-line treatment for elderly patients with multiple myeloma: the Intergroupe Francophone du Myelome 2009-01 trial (published online ahead of print). 2014 Nov 14. pii: haematol. 2014.110890.
- Niesvizky R, Martin TG 3rd, Bensinger WI, et al. Phase Ib doseescalation study (PX-171-006) of carfilzomib, lenalidomide, and lowdose dexamethasone in relapsed or progressive multiple myeloma. 2013;19(8):2248-2256.
- Shah JJ, Stadtmauer EA, Abonour R, et al. Phase I/II dose expansion of a multi-center trial of carfilzomib and pomalidomide with dexamethasone (Car-Pom-d) in patients with relapsed/refractory multiple myeloma. 2013;(653)690.
- Vesole DH, DiCapua Siegel SD, Richter JR, et al. A phase I study of carfilzomib, lenalidomide, vorinostat, and dexamethasone (QUAD) in relapsed and/or refractory multiple myeloma. 2014;32(suppl).Abstract 8535.
- Schey SA, Morgan GJ, Ramasamy K, Hazel B, Ladon D, Corderoy S, et al. The addition of cyclophosphamide to lenalidomide and dexamethasone in multiply relapsed/refractory myeloma patients; a phase I/II study. 2010;150(3):326-333.
- Lentzsch S, O’Sullivan A, Kennedy RC, et al. Combination of bendamustine, lenalidomide, and dexamethasone (BLD) in patients with relapsed or refractory multiple myeloma is feasible and highly effective: results of phase 1/2 open-label, dose escalation study. 2012;119(20):4608-4613.









