Novel ER Approaches for HR+/HER2– Breast Cancer Are Explored During SABCS
Research is ongoing to combat key resistance mechanisms, with new data recently presented by Sara A. Hurvitz, MD, FACP, and other oncologists and investigators during the 2021 San Antonio Breast Cancer Symposium.
Sara A. Hurvitz, MD

Many patients with hormone receptor-positive/ HER2-negative metastatic breast cancer (mBC) develop resistance during endocrine therapy (ET) or relapse afterward because of various mechanisms, including mutations in ESR1 (estrogen receptor 1, which encodes 1 of the 2 main types of estrogen receptors [ER]; mESR1). Most tumors remain dependent on ER signaling, and patients with disease progression may be responsive to second- or third-line ET.1,2
Research is ongoing to combat these resistance mechanisms, with new data recently presented by Sara A. Hurvitz, MD, FACP, and other oncologists and investigators during the 2021 San Antonio Breast Cancer Symposium (SABCS), held December 7-10, 2021. Results from several studies are promising and indicate that more effective therapeutic strategies are on the horizon.
“The development of resistance to endocrine-based therapy is the greatest challenge for hormone receptor–positive/HER2-negative breast cancer today,” said Hurvitz, an associate professor at the David Geffen School of Medicine, University of California, Los Angeles (UCLA) and director of the Breast Cancer Clinical Trials Program and Jonsson Comprehensive Cancer Center Clinical Research Unit at the UCLA Medical Center in Santa Monica, in an email with Targeted Therapies in Oncology™.
“Better therapies [are needed] for breast cancers resistant to endocrine therapy combined with a CDK4/6 inhibitor,” said Rachel M. Layman, MD, associate professor at The University of Texas MD Anderson Cancer Center in Houston, in a separate email with Targeted Therapies in Oncology™. “Endocrine therapy alone in this patient population has limited efficacy,” Layman added.
A mainstay for the management of hormone receptor-positive/HER2-negative metastatic breast cancer (mBC) is ET, typically with an aromatase inhibitor or fulvestrant (Faslodex) plus a CDK4/6 inhibitor (CDK4/6i).¹ ET targets ER activity and/or estrogen synthesis. CDK4/6i therapies cause cell cycle arrest and decreased expression of the proliferation biomarker Ki67 when used in conjunction with aromatase inhibitors such as anastrozole (Arimidex).²
Selective Estrogen Receptor Degraders
Elacestrant Improves Outcomes vs Standard of Care
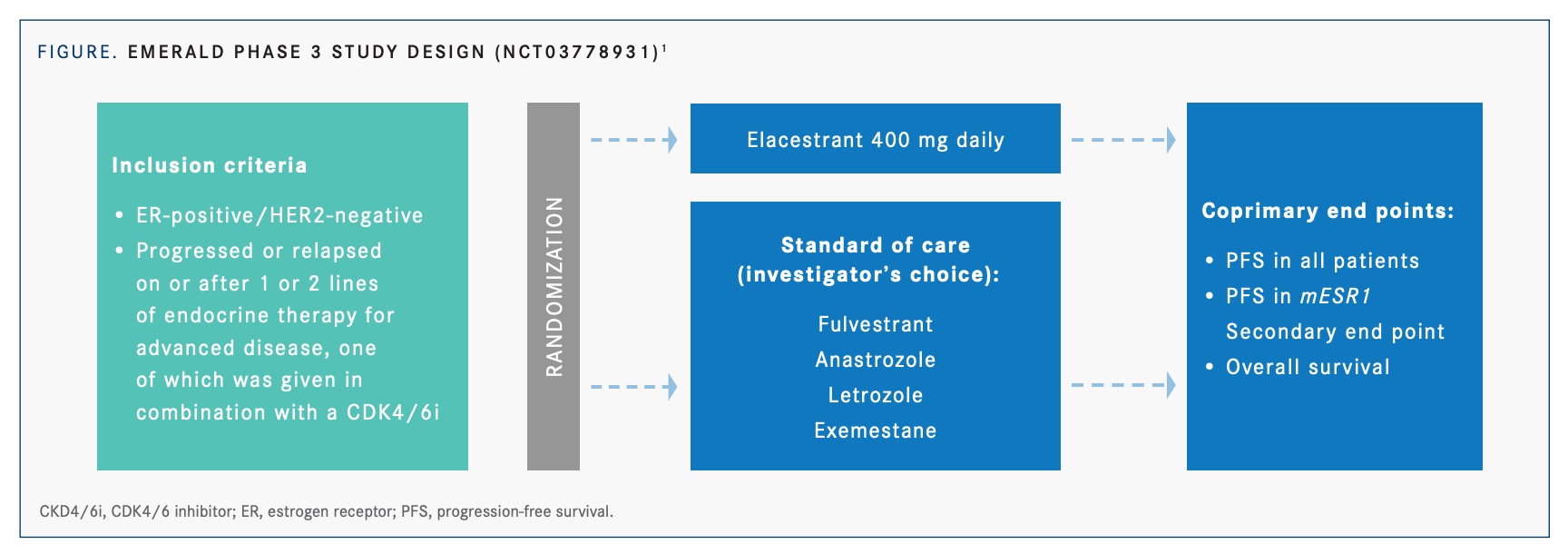
Elacestrant is considered an oral selective estrogen receptor degrader (SERD) that displays a statistically significant clinical benefit over standard-of-care therapy (SOC) in ER-positive/HER2- negative mBC in the second- or third-line setting, including tumors harboring mESR1, according to results from the phase 3, open-label EMERALD trial (NCT03778931; FIGURE1 on page 14). Enrolled patients were postmenopausal with ER-positive/ HER2-negative mBC who had previously received 1 to 2 lines of ET and less than 1 line of chemotherapy and had progression on ET plus CDK4/6i. A total of 477 patients were randomized 1:1 to receive elacestrant (400 mg orally daily) or SOC (investigator’s choice of fulvestrant or an aromatase inhibitor). The 2 primary end points were progression-free survival (PFS), by blinded independent review committee, in patients with tumors harboring mESR1 and in all patients.1
The risk of progression or death was reduced by 30% in all patients receiving elacestrant (HR, 0.697; 95% CI, 0.552-0.88; P = .0018) and by 45% in patients with mESR1 (HR, 0.546; 95% CI, 0.387- 0.786; P = .0005). The 12-month PFS rate was 22.32% (95% CI, 15.24%-29.4%) with elacestrant vs 9.42% (95% CI, 4.02%-14.81%) with SOC in all patients; the corresponding values in the mESR1 subgroup were 26.7% (95% CI, 16.17%-37.36%) vs 8.19% (95% CI, 1.26%-15.12%). An interim overall survival (OS) analysis demonstrated favorable outcomes for elacestrant compared with SOC, but results of the final OS analysis are not expected until 2023. Treatment-related adverse events (TRAEs) that were common (>10%) for patients treated with elacestrant vs SOC were mostly mild (grade 1 or 2) and included nausea (25.3% vs 8.7%), vomiting (11% vs 2.6%), and fatigue (11% vs 7.9%). Rates of grade 3 or greater AEs were 7.2% vs 3.1% for elacestrant vs SOC, respectively, and AEs leading to discontinuation were uncommon in both groups (6.3% vs 4.4%).1
The investigators reported that overall, elacestrant was well tolerated with a predictable and manageable safety profile consistent with other endocrine therapies. These results indicate that elacestrant has the potential to become the new SOC for patients with ER-positive/HER2-negative mBC. A phase 2 trial is planned to determine the impact of combining elacestrant with the CDK4/6 inhibitor abemaciclib (Verzenio) on brain metastases due to ER-positive/HER2-negative breast cancer (NCT04791384).
Neoadjuvant Giredestrant (GDC-9545) Improves Response vs Anastrozole
Results from an ongoing phase 2 trial (coopERA Breast Cancer, NCT04436744) indicate superior response to giredestrant vs anastrozole for patients with early ER-positive/HER2-negative BC. Giredestrant is a highly potent oral SERD with promising antitumor activity in mBC both as monotherapy and in combination with palbociclib (Ibrance). Patients in the coopERA Breast Cancer trial have measurable cT1c (≥ 1.5 cm)-cT4a-c ER-positive/HER2-negative, untreated, early BC and a baseline Ki67 score of 5% or more. Patients were randomized 1:1 to receive oral anastrozole (1 mg daily) or oral giredestrant (30 mg daily) for 14 days (neoadjuvant window-of-opportunity phase), followed by 16 weeks of the same daily regimen plus 125 mg oral palbociclib on days 1 to 21 of 28-day cycles (4 cycles total; neoadjuvant phase), and then surgery. The primary end point is centrally assessed geometric mean relative Ki67 score change from baseline to week 2, during the window-of-opportunity phase. This change reflects the degree to which an ET suppresses tumor cell proliferation and is a surrogate marker for clinical outcomes. Secondary end points include complete cell cycle arrest (CCCA), defined as a Ki67 score of 2.7% or less at week 2.2
Interim analysis results included 83 of the planned 202 patients. The relative reduction in the Ki67 geometric mean at 2 weeks was 80% (95% CI, –85% to –72%) with giredestrant vs 67% (95% CI, –75% to –56%; P =.0222) with anastrozole. Patients with baseline Ki67 scores of 20% or higher experienced a greater reduction with giredestrant (83%) vs anastrozole (71%), as did patients with baseline Ki67 scores less than 20% (65% giredestrant vs 24% anastrozole). AEs were less common among patients taking giredestrant (28%) vs anastrozole (38%), with no grade 3 or greater AEs or serious AEs (SAEs) related to giredestrant.2 At the end of the primary analysis, giredestrant had a significantly greater mean Ki67 reduction of 75% (95% CI, –80% to –70%) vs 67% for anastrozole (95% CI, –73% to –59%; P =.0433). The CCCA rate was 19.6% with giredestrant vs 12.8% with anastrozole (95% CI, –4.25 to 17.97), suggesting that giredestrant was better at suppressing tumor cell proliferation than anastrozole. Results of the final analysis, including overall response rates (ORRs) and combination data with palbociclib, are expected in 2022.3
“This is the first clinical trial to report a comparison between an aromatase inhibitor and an oral SERD, and it met its primary end point,” said Hurvitz. “This provides proof-of-concept data, supporting the ongoing larger phase 3 trials of giredestrant in the adjuvant setting [lidERA; NCT04961996] and [in the] metastatic setting [Persevera; NCT04546009], where clinical outcomes, not change in Ki67, will be compared between giredestrant and standard endocrine therapy.”
Giredestrant Shows Promising Activity in Advanced/Metastatic Breast Cancer
Results from the phase 1a/b GO39932 study (NCT03332797) show encouraging clinical activity of giredestrant, either as a single agent or in combination with palbociclib, in patients with locally advanced or metastatic breast cancer. Updated results of activity and biomarker analyses were presented at 2021 SABCS. Eligible patients were postmenopausal, had received up to 2 prior therapies, had experienced disease recurrence or progression while on adjuvant ET for at least 24 months, and had exhibited tumor response or stable disease for at least 6 months. One group of patients received monotherapy (30 mg oral giredestrant daily), and another received combination therapy (100 mg giredestrant plus 125 mg palbociclib, administered on a 21-days on/7- days off schedule). Treatment effects on ER signaling and tumor cell proliferation were assessed in paired pre- and on-treatment tumor biopsies using immunohistochemistry to determine biomarker protein levels of ER, progesterone receptor (PgR), and Ki67. To examine ER pathway activity, the gene expression of a predefined set of 38 ER target genes was analyzed using the Illumina TruSeq RNA Access kit. Pre- and on-treatment plasma samples were also examined for 22 mutations across the ESR1, AKT1, and PIK3CA genes in circulating tumor DNA (ctDNA) using a digital polymerase chain reaction assay.4
For patients with measurable disease at baseline, the ORRs in patients with measurable disease at baseline were 27% in the 30 mg giredestrant group (8/30 patients) and 48% in the 100 mg giredestrant plus palbociclib, with or without luteinizing hormone-releasing hormone, combination group (21/44). Clinical benefit rates were 54% (22/41 patients) and 81% (39/48), respectively. Paired biopsy analyses revealed consistent treatment-associated downregulation of ER, PgR, and Ki67 protein levels, as well as of ER pathway activity, regardless of clinical benefit or baseline mESR1. These biomarker results support consistent biological activity of giredestrant and generally consistent decreases in mESR1 ctDNA levels. Even stronger suppression of ER, PgR, and Ki67 levels was observed with the addition of palbociclib to giredestrant. Of 36 patients with ESR1 ctDNA mutations detected at baseline, 34 had decreased mESR1 ctDNA levels at the beginning of cycle 2, and 27 patients’ levels ultimately became undetectable. Early changes in ctDNA levels of PIK3CA and AKT1, but not ESR1, were correlated with response. These GO39932 trial results will encourage further investigation into the use of giredestrant in mBC.4
CDK7 Inhibition
Samuraciclib Shows Promise for Heavily Pretreated Patients
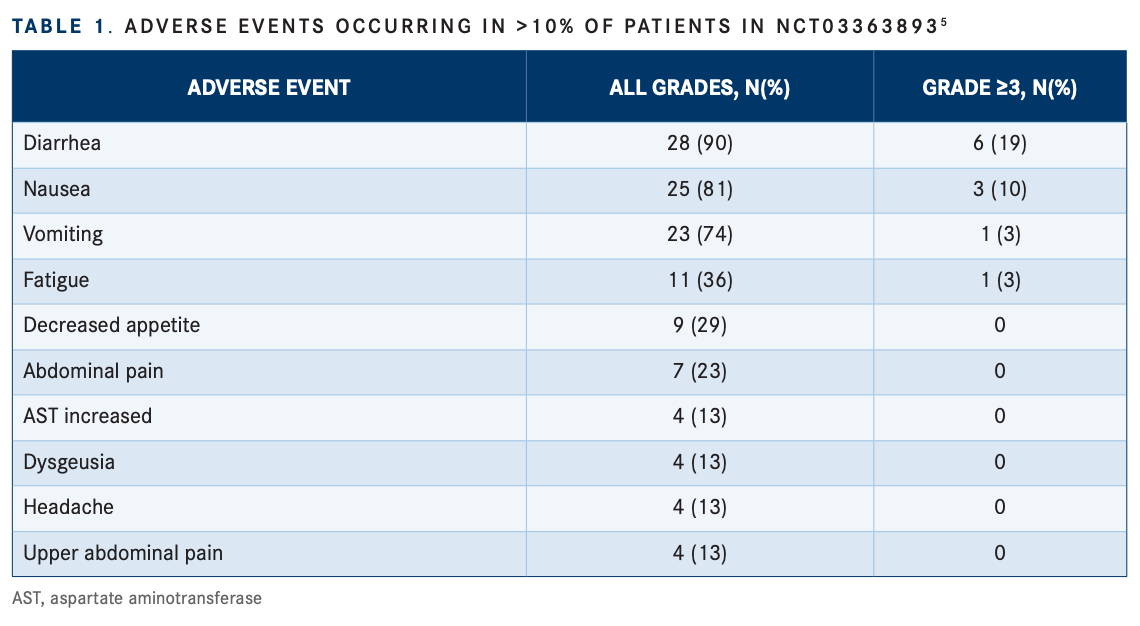
The CDK7 inhibitor samuraciclib, given in combination with fulvestrant, demonstrated antitumor activity and a tolerable safety profile for patients with advanced ER-positive BC who had progressed on previous CDK4/6i treatments, according to results from a phase 2 modular study (NCT03363893). Samuraciclib is a once-daily, oral, small-molecule, selective inhibitor of CDK7. The trial enrolled a total of 31 patients with confirmed locally advanced or metastatic ER-positive/HER2-negative BC who had prior CDK4/6i treatment, 1 or fewer lines of chemotherapy, and 2 or fewer lines of ET with no previous fulvestrant exposure.5,6
Tumor shrinkage was observed in 72% (18/25) of evaluable patients. Stratification of patients according to TP53 status showed that the 19 patients (76%) with wild-type TP53 displayed a median PFS of 32 weeks compared with 7.9 weeks for the 6 patients with mutant TP53 (HR, 0.17; 95% CI, 0.05-0.53; P =.0008). The CBR at 24 weeks was 36% overall, 55% in patients without liver metastases, and 53% in patients with wild-type TP53. Liver metastasis at baseline, a known risk factor for poor prognosis, negatively impacted median PFS, which was not reached in the 17 patients without liver metastasis and was 11.9 weeks in the 14 patients with liver metastasis (HR, 0.16; 95% CI, 0.05-0.59; P =.0021). The predictive value of liver metastasis on samuraciclib therapy will be evaluated in future studies. The most common AEs were reversible, low-grade gastrointestinal events, including diarrhea (90%), nausea (81%), vomiting (74%), and fatigue (36%). Grade 3 or higher AEs were uncommon and included diarrhea (19%) and nausea (10%; TABLE 15 ).6
Samuraciclib has been granted fast track designation by the FDA for treatment of HR-positive, HER2-negative advanced BC and locally advanced or metastatic triple-negative breast cancer (TNBC).7
Antibody-Drug Conjugates
Datopotamab Deruxtecan Promising for Triple-Negative Breast Cancer
Datopotamab deruxtecan (Dato-DXd) demonstrated promising antitumor activity with a manageable safety profile in patients with previously treated advanced or metastatic TNBC, according to results from the phase 1 TROPION-PanTumor01 study (NCT03401385). Datopotamab deruxtecan is an antibody-drug conjugate (ADC) consisting of a humanized anti-TROP2 IgG1 monoclonal antibody linked to a topoisomerase I inhibitor. A total of 44 patients were included in the TNBC cohort. Based on dose-escalation studies in the non–small cell lung cancer cohort, a dose of 6 mg/kg intravenously every 3 weeks was recommended; however, 2 patients with TNBC received 8 mg/kg prior to this dose selection.
Many patients were heavily pretreated, with 68% having received 2 or more prior lines of therapy. The ORR among 38 evaluable patients was 34% (14 patients with confirmed complete [CR] or partial response [PR] and 1 patient with confirmation pending). Stable disease was observed in 17 patients (39%), 2 patients (5%) were not evaluable, and 8 patients (18%) had progressive disease. For patients who had not received prior treatment with a topo I inhibitor– based ADC (n=27), the ORR was 52% at a median follow-up of 8.8 months. This included 13 patients (48%) with confirmed CR or PR, 1 patient (4%) with confirmation pending, 9 patients (33%) with SD, 1 patient (4%) who was not evaluable, and 4 patients (15%) with progressive disease. Treatment-emergent AEs (TEAEs) occurred in 98% of patients, with 45% being grade 3 or higher, and 23% of patients experienced grade 3 or higher TEAEs.9
The HER2-negative cohort of TROPION-PanTumor01 is fully enrolled, and data are forthcoming. The phase 1b/2 BEGONIA trial (NCT03742102) is also underway; it is evaluating datopotamab deruxtecan in combination with durvalumab (Imfinzi) as a first-line treatment for patients with metastatic TNBC. The phase 3 TRIOPION-Breast01 trial is evaluating datopotamab deruxtecan in HR-positive/HER2-negative breast cancer, and a phase 3 trial in patients with TNBC is being planned.9
Other Novel HER2- Negative Treatments
Balixafortide Fails to Improve
Results in Combination With Eribulin Results from the international, phase 3 FORTRESS trial (NCT03786094) indicate that the combination of balixafortide and the microtubule inhibitor eribulin (Halaven) has no advantage over eribulin alone for treating patients with HER2-negative mBC. Balixafortide is a synthetic peptide and a potent selective CXCR4 antagonist. The phase 1 FORTRESS trial (NCT01837095) evaluating balixafortide combined with eribulin in patients with HER2-negative mBC provided promising efficacy and safety data. Patients had HER2-negative, locally advanced or metastatic BC and had received 1 to 4 lines of chemotherapy in the advanced setting. A total of 432 patients were enrolled, 348 of whom were treated in the third-line setting.10
Median PFS was not improved by the addition of balixafortide to eribulin (3.5 months vs 4.0 months for eribulin monotherapy; HR, 1.07; 96% CI, 0.81-1.41), nor was interim median OS (11.0 months vs 11.2 months; HR, 1.08; 99.9% CI, 0.66-1.77). Similarities between the arms were also observed in the safety population regarding serious TEAEs (28% vs 26.5%), TEAEs leading to death (6.9% vs 6.4%), and TEAEs of grade 3 or higher (68.3% vs 64.2%).10
Gedatolisib Displays Antitumor Activity in First and Late-line Settings
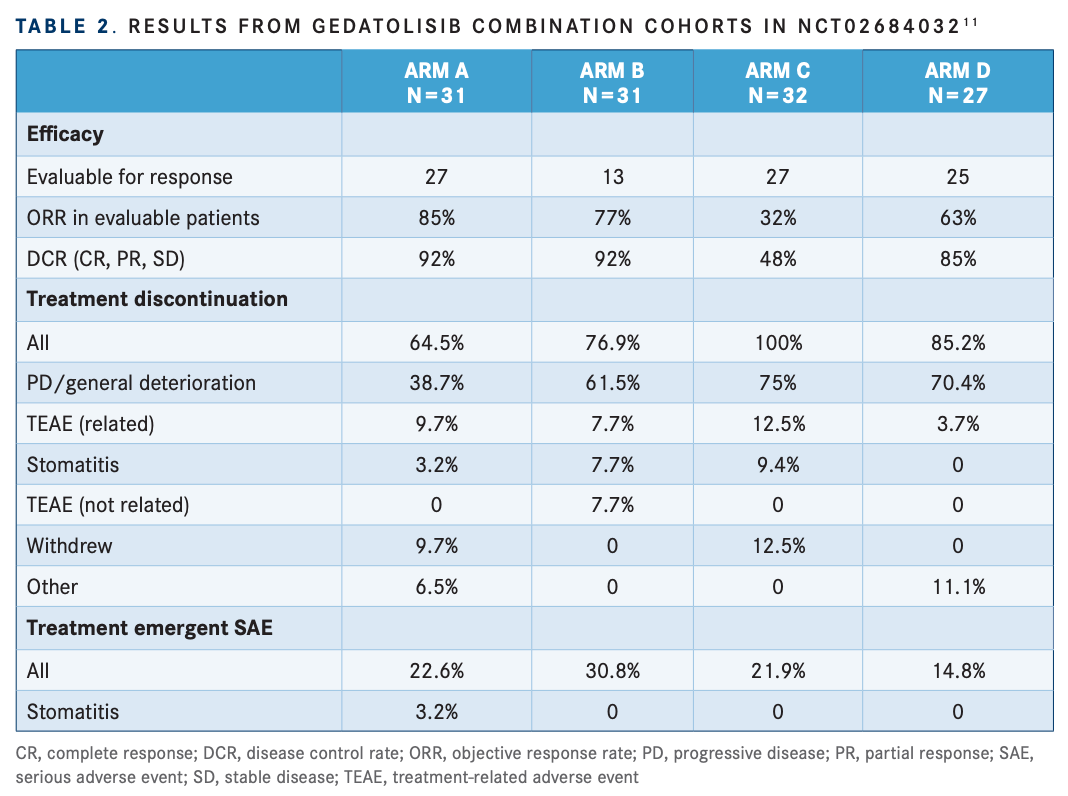
According to results from a phase 1b expansion study (NCT02684032), the addition of gedatolisib to palbociclib and ET (letrozole; Femara) or fulvestrant) in women with ER-positive/HER2-negative mBC provided promising antitumor activity in both first- and later-line settings compared with historical controls. Gedatolisib is a dual inhibitor of PI3K and mTOR. A total of 103 patients were enrolled in one of 4 arms: A) gedatolisib, palbociclib, and letrozole as first-line treatment in CDK4/6inaïve patients (n = 31); B) gedatolisib,palbociclib, and fulvestrant as second-line treatment in CDK4/6i-naïve patients (n=13); C) gedatolisib, palbociclib, and fulvestrant as second- or third-line treatment in patients with prior CDK4/6i therapy (n=32); or D) gedatolisib, palbociclib, and fulvestrant as second- or third-line treatment in patients whose most recent regimen included a CDK4/6i (n = 27).11
Results are summarized in TABLE 211. Median PFS was not reached, 31.1 months, 11.9 months, 5.1 months, and 12.9 months in arms A, B, C, and D, respectively. The most common TEAEs (≥ 50%) across all arms were nausea (81.6%), stomatitis (80.6%), fatigue (79.6%), and neutropenia/neutrophil count decrease (79.6%). The most common treatment-emergent SAEs (≥ 2%) were febrile neutropenia (4.9%), acute kidney injury (2.9%), and pyrexia (2.9%).11
“The treatment regimen was very effective for metastatic HR-positive/ HER2-negative breast cancer, especially when compared with standard-of-care treatments currently available,” Layman said. “Results also suggest that the treatment may be effective in patients with harder to treat breast cancers, such as those with primary endocrine resistance.” A phase 3 study evaluating gedatolisib in patients with ER-positive/ HER2-negative mBC is planned.
Looking Ahead
Several therapies presented at 2021 SABCS hold much promise for patients with hormone receptor–positive/HER2-negative BC, and oncologists are eager to see further results in the upcoming year. Hurvitz is keeping a close eye on SERDs and ADCs. “EMERALD data are exciting, and data from ongoing studies evaluating other ER degraders are going to be very interesting. [Further] studies evaluating antibody-drug conjugates in hormone receptor–positive/HER2-negative breast cancer are highly anticipated.”
Layman is also watching the developing SERDs closely, along with PROTACs (proteolysis Targeting Chimera). “SERDs and PROTACs are showing promising clinical trial results and may become available as new treatment options. This is great news for patients with hormone receptor–positive, HER2-negative breast cancer.”
REFERENCES:
1. Bardia A, Neven P, Streich G, et al. Elacestrant, an oral selective estrogen receptor degrader (SERD), vs investigator’s choice of endocrine monotherapy for ER-positive/HER2-negative advanced/metastatic breast cancer (mBC) following progression on prior endocrine and CDK4/6 inhibitor therapy: results ofEMERALD phase 3 trial. presented at: San Antonio Breast Cancer Symposium; December 8, 2021 2021; San Antonio, TX. Session GS2.Accessed January 6, 2022https://bit.ly/33hrpIg
2. Hurvitz S, Quiroga V, Hee Park Y, et al. Neoadjuvant giredestrant (gdc-9545) + palbociclib versus anastrozole + palbociclib in postmenopausal women with estrogen receptor-positive, her2-negative, untreated early breast cancer: primary analysis of the randomized, open-label, phaseII coopERA breast cancer study. Presented at: San Antonio Breast Cancer Symposium; December 10, 2021 2021; San Antonio, TX. Session PD13. https://www.abstractsonline.com/pp8/#!/10462/presentation/594
3. Investor Update. Roche; December 10, 2021, 2021. https://www.roche.com/investors/updates/inv-update-2021-12-10b.htm
4. Turner N, Loi S, Moore H, et al. Activity and biomarker analyses from a phase Ia/b study of giredestrant(GDC-9545; G) with or without palbociclib (palbo) in patients with estrogen receptor-positive, HER2-negative locally advanced/metastatic breast cancer (ER-positive/HER2-negativeLA/mBC). Presented at: San Antonio Breast Cancer Symposium; December 10, 2021 2021; San Antonio, TX. Session PD13. https://www.abstractsonline.com/pp8/#!/10462/presentation/595
5. Coombes C, Howell SJ, Krebs MG, et al. Study of samuraciclib (ct7001), a first-in-class, oral, selective inhibitor of cdk7, in combination with fulvestrant in patients with advanced hormone receptor positive her2 negative breast cancer(HR+BC). Presented at: San Antonio Breast Cancer Symposium; December 9, 2021 2021; San Antonio, TX. Session GS3. https://www.abstractsonline.com/pp8/#!/10462/presentation/2079
6. Pizii W. Samuraciclib plus fulvestrant improves PFS in heavily pretreated HR+ breast cancer. Updated December 10, 2021. Accessed January 4, 2022, 2022. https://www.onclive.com/view/samuraciclib-plus-fulvestrant-improves-pfs-in-heavily-pretreated-hr-breast-cancer
7. 8. Carrick Therapeutics Receives FDA fast track designations for two samuraciclib combinations for the treatment of HR+, HER2-advanced breast cancer and locally advanced or metastatic triple negative breast cancer. August 16, 2021, 2021. https://bit.ly/3JFs9HL
9. Krop I, Juric D, Shimizu T, et al. Datopotamab deruxtecanin advanced/metastatic her2-breast cancer: results from the phase 1 TROPION-PanTumor01 study. Presented at: San Antionio Breast Cancer Symposium; December 7, 2021 2021; San Antonio, TX. Session GS1. https://www.abstractsonline.com/pp8/#!/10462/presentation/2164
10. Writer S. Datopotamab deruxtecan demonstrates highly promising efficacy in advanced or metastatic TNBC. Updated December 7, 2021. Accessed January 4, 2022, 2022. https://www.targetedonc.com/view/datopotamab-deruxtecan-demonstrates-highly-promising-efficacy-in-advanced-or-metastatic-tnbc
11. Kaufman P, Martin M, Mayer I, et al. Balixafortide (a CXCR4 antagonist)+eribulin versus eribulin alone in patients with her2 negative, locally recurrent or metastatic breast cancer: an international, randomized, phase 3 trial (FORTRESS). Presented at: San Antonio Breast Cancer Symposium; December 10, 2021 2021; San Antonio, TX. Session PD13. https://www.abstractsonline.com/pp8/#!/10462/presentation/589
12. Layman R, Wesolowski R, Han H, et al. Phase Ib expansion study of gedatolisib in combination with palbociclib and endocrine therapy in women withER+ metastatic breast cancer. Presented at: San Antonio Breast Cancer Symposium; 2021; San Antonio, TX. Session PD13. https://www.abstractsonline.com/pp8/#!/10462/presentation/590
