New Mechanisms Under Study to Overcome Osimertinib Resistance in EGFR+ NSCLC
Although osimertinib has been shown to overcome T790M-mediated mutations in the second-line setting, as with other EGFR tyrosine kinase inhibiors progression on osimertinib is inevitable.
Adrian G. Sacher, MD, MMSc, FRCPC

Several small molecule EGFR tyrosine kinase inhibitors (TKIs) are now available for the treatment of EGFR-mutated non–small cell lung cancer (NSCLC). However, progression after TKI therapy—including with the third-generation TKI osimertinib (Tagrisso)—is inevitable. Results of several studies targeting new treatment strategies were reported at the recent International Association for the Study of Lung Cancer 2021 World Congress on Lung Cancer (WCLC).
Approximately 10% to 15% of patients with NSCLC in the United States and Europe and 45% of patients with NSCLC in Asia have disease with EGFR-sensitizing mutations.1 The most common sensitizing mutations are deletions in exon 19 (45%) and a point mutation in exon 21 (L858R, 40%). Tumors with these mutations initially respond to EGFR TKIs, including erlotinib (Tarceva), gefitinib (Iressa), afatinib (Gilotrif), osimertinib, and dacomitinib (Vizimpro), but they ultimately develop resistance mutations and stop responding to EGFR TKIs, usually within months.2,3
Mechanisms of Resistance
The most common mechanism of acquired resistance is the development of a second mutation in exon 20 of EGFR, known as T790M.4 EGFR T790M develops in approximately 60% of cases of NSCLC upon treatment with TKIs5 and decreases the affinity of EGFR-TKI binding to the adenosine triphosphate binding pocket of EGFR.6
Osimertinib is a third-generation EGFR TKI that targets both sensitizing and resistant T790M mutations.7 According to National Comprehensive Cancer Network guidelines, osimertinib is the preferred first-line EGFR TKI option for patients with EGFR-positive metastatic disease.2 In addition, osimertinib is indicated for adult patients with EGFR T790M mutation–positive NSCLC whose disease has progressed on or after EGFR TKI therapy.8 Although osimertinib has been shown to overcome T790M-mediated mutations in the second-line setting,9 as with other EGFR TKIs progression on osimertinib is inevitable.
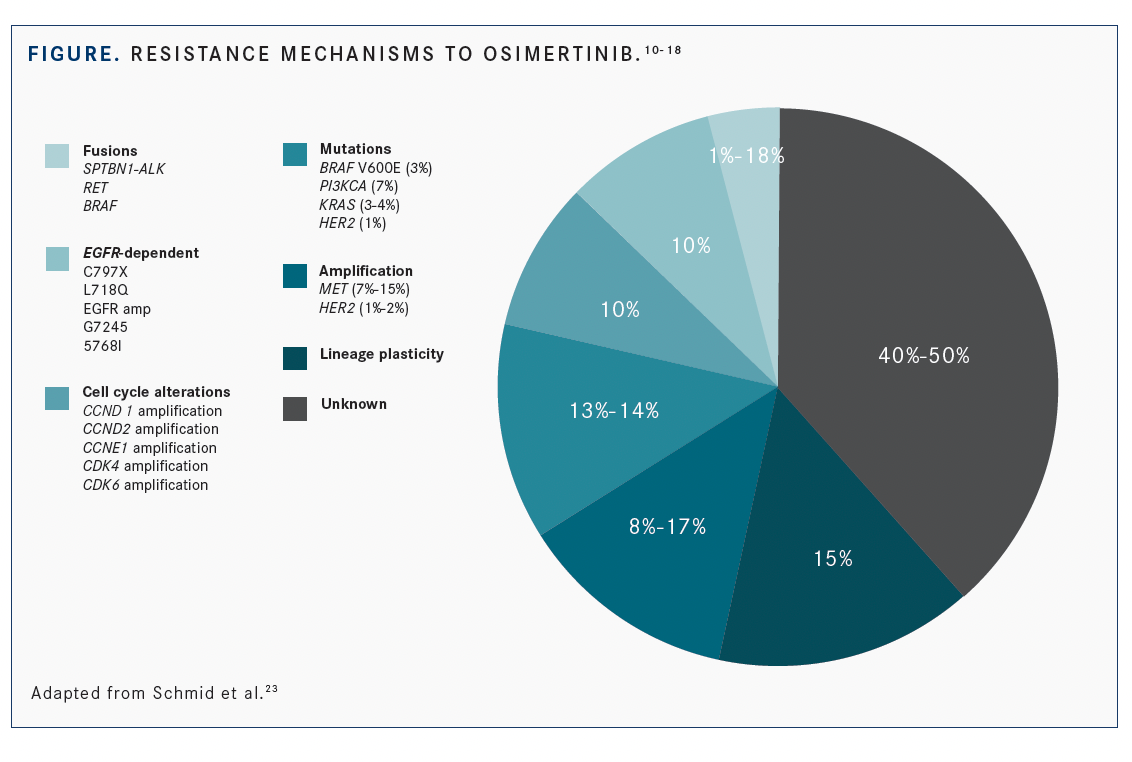
In addition to T790M, other EGFR-dependent pathways of osimertinib resistance include new development of EGFR C797S mutations and multiple others, including L792X, G796S, L718Q, S768I, G796R, G796D, and G724S (FIGURE).10-18 EGFR amplification and copy number alteration also are EGFR-dependent mechanisms for the secondary drug resistance of osimertinib.4,19,20 EGFR-independent mechanisms of resistance include MET amplification, which accounts for 5% to 10% of acquired EGFR-TKI resistance cases,10,21 HER2 amplification, and KRAS and PIK3CA gene mutations.22 Currently, approximately 50% of resistance mutations are unknown.23
Patterns of Disease Resistance on Osimertinib
Results of several studies presented at the 2021 WCLC attempted to characterize the patterns of disease progression on osimertinib and potential strategies for improving duration of progression-free survival (PFS) and overall survival (OS) with combination approaches including osimertinib.
With the goal of examining patterns of disease progression on osimertinib, Hye Sook Kim, MD, of Inje University College of Medicine, Ilsan Paik Hospital, in Goyang, South Korea, and colleagues conducted a retrospective review of 98 records of patients who were undergoing initial or subsequent treatment with osimertinib.24
“Little is known about patterns of treatment failure on osimertinib and subsequent treatment in NSCLC following progression with osimertinib in the real-world setting,” Kim noted during the presentation.
The investigators found that approximately 80% of patients on osimertinib developed systemic progressive disease, whereas 20% developed more limited progressive disease, defined as progression in 5 or fewer lesions and 3 or fewer organs, with other lesions in a controlled state.
They also found that disease progression typically occurs outside the central nervous system (CNS) in preexisting thoracic lesions or organs and that new brain metastases were rare, occurring in approximately 16% of patients.
Approximately 27% of patients experiencing progressive disease with osimertinib continued osimertinib maintenance, the analysis found; approximately half (51.4%) received cytotoxic chemotherapy; and the remainder received radiation (14.3%), another EGFR inhibitor (4.3%), or a checkpoint inhibitor (2.9%).
Results of another study of patients receiving osimertinib with leptomeningeal metastases (LM), presented by Di Zheng, MD, chief physician at the Department of Medical Oncology, Shanghai Pulmonary Hospital, Tongji University, China, concluded that evaluating circulating free (cf) DNA for resistance mechanisms to osimertinib in cerebrospinal fluid (CSF) may be useful in matching ongoing targeted therapy, especially if druggable targets are present.25
Of the patients, 22 had treatment matched to resistance mutations present in cfDNA and 59 did not. The 22 patients with matched treatment had an OS of 9.8 months (95% CI, 7.2-12.4). In the 59 patients who did not receive matched therapy, osimertinib plus other therapy (chemotherapy/bevacizumab [Avastin]/radiotherapy) resulted in the greatest benefit, with a 14.3-month median OS. The investigators also concluded that dose intensification (eg, 160 mg/day, twice the usual dose) of osimertinib might prolong survival of patients with LM.
According to Zheng, use “of CSF for detecting resistant mechanisms to osimertinib seems feasible and may provide therapeutic benefit for patients with LM and druggable targets.”
Combinations With Osimertinib After Disease Progression
Several ongoing studies are exploring new treatment options after disease progression on osimertinib. According to Adrian G. Sacher, MD, MMSc, FRCPC, a thoracic oncologist and affiliate scientist at the Princess Margaret Cancer Centre and an assistant professor at the University of Toronto in Canada, osimertinib provides excellent penetrance into the CNS and favorable tolerance. It currently represents the standard of care for the initial treatment of EGFR-mutated metastatic NSCLC.26 During his overview presentation of novel osimertinib combinations in EGFR-mutated NSCLC, he added that potential osimertinib combinations include osimertinib plus other targeted therapies and some types of chemotherapies with distinct mechanisms of action, but he noted that the “likelihood of success hinges on rational selection of the study population including consideration of genomic biomarkers.”
Pelcitoclax and Osimertinib
One multicenter phase 1b clinical trial (NCT04001777) evaluated osimertinib in combination with pelcitoclax (APG-1252).27 Pelcitoclax is a dual BCL-2/BCL-xL inhibitor that has demonstrated preclinical inhibition of tumor growth and synergistic anti-tumor effects with osimertinib as well as potentially less platelet destruction than navitoclax.
This study included a dose-escalation phase and a dose-expansion phase. Pelcitoclax was administered at 160 mg or 240 mg intravenously once a week, and osimertinib was administered orally at 80 mg daily. The dose-escalation phase was designed to evaluate the safety and tolerability of the combination regimen in patients who progressed on prior EGFR TKI treatments as well as to find the recommended phase 2 dose for the combination. The subsequent dose-expansion phase consisted of 2 arms, one with patients with third-generation EGFR TKI–resistant NSCLC, and the other with patients with NSCLC who had not received osimertinib.
In the dose-escalation phase, 1 partial response (PR) was observed among 11 evaluable patients. In the first arm of the dose-expansion phase, 3 patients achieved PRs (2 with osimertinib-resistant disease) and 13 had stable disease (SD) among 20 evaluable patients, for an objective response rate (ORR) of 15% and a disease control rate (DCR) of 80%. In the second arm of the dose-expansion phase, 13 PRs and 8 SDs were observed among 22 evaluable patients, including 3 patients harboring an EGFR exon 20 insertion, for an ORR of 59.1% and a DCR of 95.5%.
The most common treatment-related adverse events with the regimen included transient thrombocytopenia, increased aspartate aminotransferase and alanine aminotransferase, increased amylase, increased blood creatinine, decreased leukocytes, anemia, and rash. One case of dose-limiting grade 4 thrombocytopenia was observed with 240 mg pelcitoclax during the dose-escalation phase. Pelcitoclax 160 mg per week plus osimertinib 80 mg once daily was selected as the recommended phase 2 dose.
According to the presenter Li Zhang, MD, of the State Key Laboratory in the South China Collaborative Innovation Center for Cancer Medicine, Sun Yat-Sen University Cancer Center, in Guangzhou, combination treatment with pelcitoclax and osimertinib at the recommended phase 2 dose was safe and well tolerated.
He noted that “preliminary efficacy of this combination was observed in some patients with osimertinib resistance.”
Afatinib and Osimertinib
Another phase 1 study evaluated afatinib in combination with osimertinib in patients who failed prior osimertinib.28 According to Satoru Miura, MD, PhD, with the Department of Internal Medicine of the Niigata Cancer Center Hospital in Japan, “concurrent use of afatinib with osimertinib may have the potential to eradicate not only secondary resistant clones but also co-existing uncommon EGFR mutation clones.”
The study included 13 patients, who received 20 mg/day (n = 6) or 30 mg/day (n = 7) afatinib, both doses in combination with 80 mg/day osimertinib. Dose-limiting toxicities included 1 case of grade 3 diarrhea with 20 mg/day afatinib as well as 2 cases of grade 2 nausea/ vomiting and 1 case of grade 3 diarrhea with 30 mg/day afatinib. The ORR was 7.7% (95% CI, 0.2%-36.0%) and the DCR was 46.2% (95% CI, 19.2%-74.9%). The median PFS was 2.4 months (95% CI, 1.4–not reached [NR]) and the median OS was 25.4 months (95% CI, 4.6–NR). According to Miura, the recommended dose for further study was 20 mg/day afatinib due to a high rate of dose-limiting toxicity with the maximum tolerated dose of 30 mg/day.
Ado-Trastuzumab Emtansine and Osimertinib
Merel Jebbink, MD, with the Department of Thoracic Oncology at the Netherlands Cancer Institute in Amsterdam, reported interim data from the phase 2 TRAEMOS study (NCT03784599), the first to combine ado-trastuzumab emtansine (Kadcyla; T-DM1) and osimertinib in patients with EGFR-mutated NSCLC.29
In the population evaluable for efficacy and safety (n = 27), the ORR was 11% (n = 3) with a DCR of 48% (n = 13) after 12 weeks; the median PFS was 2.7 months (95% CI, 2.1-3.5).
According to Jebbink, the findings suggested that the combination of T-DM1 and osimertinib, while demonstrating a favorable safety profile compared with cytotoxic chemotherapy, resulted in limited efficacy in this setting and does not warrant further investigation. However, Sacher noted in his overview presentation discussing this trial that this combination may have promise in patients with HER2-amplified/mutated disease. Sacher added that “there must be a clear focus on a single treatment context [ie, acquired resistance vs first line]” and there must be care taken to “avoid synergistic toxicity, which can be problematic and dose limiting.”26
Pembrolizumab and Platinum-Based Chemotherapy After Osimertinib
A phase 2 study, also reported at WCLC by Shirish Gadgeel, MD, a professor in the Department of Internal Medicine and head of the Division of Hematology and Oncology, Henry Ford Health System in Detroit, Michigan, and colleagues, explored treatment with pembrolizumab (Keytruda) in combination with chemotherapy (carboplatin and pemetrexed) followed by 2 years of maintenance pemetrexed and pembrolizumab among patients with recurrent EGFR-mutated (exon 19 deletion; exon 21 L858R, L861Q; exon 18 G719X, or S768I) NSCLC. The treatment regimen demonstrated a response rate of 42.3% (95% CI, 23%-63%), a median PFS of 8.3 months (95% CI, 7.2-16.5), and median OS of 22.2 months (95% CI, 20.6–not evaluable).30 Of the 26 patients with EGFR mutations, 22 (85%) had received prior osimertinib. The 12-month OS rate was 76% (95% CI, 59%-97%), and the 12-month PFS rate was 29% (95% CI, 14%-59%).
According to the investigators, these results warranted further study of the combination. However, the study closed early due to slow enrollment.
Ongoing Studies
Several ongoing studies may provide additional insights into treatment strategies for patients progressing on osimertinib. The phase 2 ORCHARD study (NCT03944772) will include an estimated 150 participants with advanced NSCLC who progressed on first-line osimertinib therapy. Patients will be divided into groups and will receive osimertinib in combination with one of several agents, including savolitinib, gefitinib, necitumumab (Portrazza), pemetrexed plus durvalumab (Imfinzi), alectinib (Alecensa), and selpercatinib (Retevmo). Primary end point is ORR measured over an average of 3 months.
The INSIGHT 2 study (NCT03940703) is also underway to evaluate tepotinib (Tepmetko) in combination with osimertinib.31 Tepotinib is a highly selective and potent TKI to MET, which is amplified in approximately 30% of patients who progress on EGFR TKIs. The study is anticipated to enroll 120 patients. Primary end point is ORR in patients with MET amplification; centrally confirmed by FISH. Secondary end points include ORR by investigator assessment, duration of response, DCR, PFS, OS, pharmacokinetics, health-related quality of life, tolerability, and safety.
The question also remains whether osimertinib should be continued with chemotherapy upon progression, although current guidelines recommend discontinuation of osimertinib upon systemic progression and initiation of platinum-based doublet chemotherapy.2 According to Lecia V. Sequist, MD, MPH, director of the Center for Innovation in Early Cancer Detection at Massachusetts General Hospital in Boston, and colleagues, “continued osimertinib treatment during chemotherapy may be beneficial compared with chemotherapy alone, particularly in patients with CNS metastases, given osimertinib has demonstrated superior CNS efficacy compared with chemotherapy.” In their abstract describing the phase 3 COMPEL trial (NCT04765059), they note that continuing osimertinib may prevent a rebound phenomenon.31 This trial will include more than 200 patients with EGFR-mutated (Ex19del/L858R) locally advanced, metastatic or recurrent NSCLC randomized to 1 of 2 arms. Patients in 1 arm will receive pemetrexed plus cisplatin or carboplatin plus osimertinib 80 mg, followed by pemetrexed maintenance 500 mg/m2 and osimertinib 80 mg. Patients in the other arm will receive the same chemotherapy plus placebo in place of osimertinib, followed by pemetrexed maintenance and placebo. The primary end point is PFS. Results are expected in September 2024.
Conclusions
Although multiple TKIs are approved for EGFR-mutated NSCLC, development of resistance to these agents for now is inevitable and approximately half the resistance mutations involved in progression are unknown. Several new and emerging combinations of therapies hold promise for improved outcomes in EGFR-mutated NSCLC. Collectively, study results suggest that upon disease progression with osimertinib, patients should be retested for mutations that may be targeted for therapeutic benefit. As the understanding of precision medicine in oncology evolves, treatment selection based on resistance mutations will become increasingly important, both before initial treatment and upon progression.
References:
1. Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015;5(9):2892-2911.
2. NCCN. Clinical Practice Guidelines in Oncology. Non-small cell lung cancer, version 6.2021. Accessed October 2, 2021. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
3. Langer CJ. Epidermal growth factor receptor inhibition in mutation-positive non-small-cell lung cancer: is afatinib better or simply newer? J Clin Oncol. 2013;31(27):3303-3306. doi:10.1200/JCO.2013.49.8782
4. Qu F, Zhou Y, Yu W. A review of research progress on mechanisms and overcoming strategies of acquired osimertinib resistance. Anticancer Drugs. Published online September 13, 2021. doi:10.1097/CAD.0000000000001242
5. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240-2247. doi:10.1158/1078-0432.CCR-12-2246
6. Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008;105(6):2070-2075. doi:10.1073/pnas.0709662105
7. Cross DAE, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4(9):1046-1061. doi:10.1158/2159-8290.CD-14-0337
8. Tagrisso (osimertinib). Prescribing information. AstraZeneca; 2021. Accessed October 1, 2021. https://bit.ly/3iuDJtc
9. Mok TS, Wu YL, Ahn MJ, et al; AURA3 Investigators. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629-640. doi:10.1056/NEJMoa1612674
10. Papadimitrakopoulou VA, Wu YL, Han JY, et al. Analysis of resistance mechanisms to osimertinib in patients with EGFR T790M advanced NSCLC from the AURA3 study. Ann Oncol. 2018;29(suppl 8):LBA51. doi:10.1093/annonc/mdy424.064
11. Ramalingam SS, Cheng Y, Zhou C, et al. Mechanisms of acquired resistance to first-line osimertinib: preliminary data from the phase III FLAURA study. Ann Oncol. 2018;29(suppl 8):LBA50. doi:10.1093/annonc/mdy424.063
12. Yang Z, Yang N, Ou Q, et al. Investigating novel resistance mechanisms to third-generation EGFR tyrosine kinase inhibitor osimertinib in non-small cell lung cancer patients. Clin Cancer Res. 2018;24(13):3097-3107. doi:10.1158/1078-0432.CCR-17-2310
13. Ou SI, Cui J, Schrock AB, et al. Emergence of novel and dominant acquired EGFR solvent-front mutations at Gly796 (G796S/R) together with C797S/R and L792F/H mutations in one EGFR (L858R/T790M) NSCLC patient who progressed on osimertinib. Lung Cancer. 2017;108:228-231. doi:10.1016/j.lungcan.2017.04.003
14. Zheng D, Hu M, Bai Y, et al. EGFR G796D mutation mediates resistance to osimertinib. Oncotarget. 2017;8(30):49671-49679. doi:10.18632/oncotarget.17913
15. Ercan D, Choi HG, Yun CH, et al. EGFR mutations and resistance to irreversible pyrimidine-based EGFR inhibitors. Clin Cancer Res. 2015;21(17):3913-3923. doi:10.1158/1078-0432.CCR-14-2789
16. Oztan A, Fischer S, Schrock AB, et al. Emergence of EGFR G724S mutation in EGFR-mutant lung adenocarcinoma post progression on osimertinib. Lung Cancer. 2017;111:84-87. doi:10.1016/j.lungcan.2017.07.002
17. Fassunke J, Müller F, Keul M, et al. Overcoming EGFRG724S-mediated osimertinib resistance through unique binding characteristics of second-generation EGFR inhibitors. Nat Commun. 2018;9(1):4655. doi:10.1038/s41467-018-07078-0
18. Bersanelli M, Minari R, Bordi P, et al. L718Q mutation as new mechanism of acquired resistance to AZD9291 in EGFR-mutated NSCLC. J Thorac Oncol. 2016;11(10):e121-e123. doi:10.1016/j.jtho.2016.05.019
19. Thress KS, Paweletz CP, Felip E, et al. Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med. 2015;21(6):560-562. doi:10.1038/nm.3854
20. Oxnard GR, Hu Y, Mileham KF, et al. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M-positive lung cancer and acquired resistance to osimertinib. JAMA Oncol. 2018;4(11):1527-1534. doi:10.1001/jamaoncol.2018.2969
21. Planchard D, Loriot Y, André F, et al. EGFR-independent mechanisms of acquired resistance to AZD9291 in EGFR T790M-positive NSCLC patients. Ann Oncol. 2015;26(10):2073-2078. doi:10.1093/annonc/mdv319
22. Buttitta F, Felicioni L, Di Lorito A, et al. Early prediction of resistance to tyrosine kinase inhibitors by plasma monitoring of EGFR mutations in NSCLC: a new algorithm for patient selection and personalized treatment. Oncotarget. 2020;11(11):982-991. doi:10.18632/oncotarget.27517
23. Schmid S, Li JJN, Leighl NB. Mechanisms of osimertinib resistance and emerging treatment options. Lung Cancer. 2020;147:123-129. doi:10.1016/j.lungcan.2020.07.014
24. Kim HS, Han JY. Pattern of disease progression on osimertinib and subsequent treatment in patients with EGFR-mutated NSCLC. Presented at: IASLC 2021 World Conference on Lung Cancer; September 8-14, 2021; virtual. Abstract P51.04.
25. Zheng MM, Li YS, Tu HY, et al. Matched targeted therapy by cfDNA of CSF beyond leptomeningeal metastases progression upon osimertinib in patients with EGFR mutated NSCLC. Presented at: IASLC 2021 World Conference on Lung Cancer; September 8-14, 2021; virtual. Abstract OA116.03.
26. Sacher AG. Novel osimertinib combinations in EGFR mutant mNSCLC. Presented at: IASLC 2021 World Conference on Lung Cancer; September 8-14, 2021; virtual. Abstract MA02.08.
27. Zhang L, Zhao H, Ma Y, et al. Phase 1b study of pelcitoclax (APG 1252) in combination with osimertinib in patients with EGFR TKI resistant NSCLC. Presented at: IASLC 2021 World Conference on Lung Cancer; September 8-14, 2021; virtual. Abstract MA02.06.
28. Miura S, Azuma K, Yoshioka H, et al. A phase I study of afatinib in combination with osimertinib. Presented at: IASLC 2021 World Conference on Lung Cancer; September 8-14, 2021; virtual. Abstract MA02.05.
29. Jebbink M, de Langen AJ, Monkhorst K, et al. Trastuzumab emtansine and osimertinib (TRAEMOS) combination treatment to target HER2 bypass track resistance in EGFR mutation positive NSCLC: interim analysis of a phase II trial. Presented at: IASLC 2021 World Conference on Lung Cancer; September 8-14, 2021; virtual. Abstract MA02.07.
30. Gadgeel S, Dziubek K, Nagasaka M, et al. Pembrolizumab in combination with platinum-based chemotherapy in recurrent EGFR/ALK-positive non–small cell lung cancer (NSCLC). Presented at: IASLC 2021 World Conference on Lung Cancer; September 8-14, 2021; virtual. Abstract OA09.03
31. Dooms C, Nadal E, Raskin J, et al. Tepotinib + osimertinib for EGFR-mutant NSCLC with resistance to first-line osimertinib due to MET amplification: INSIGHT 2. Presented at: IASLC 2021 World Conference on Lung Cancer; September 8-14, 2021; virtual. Abstract P47.09.
32. Sequist LV, Peled N, Tufman A, et al. COMPEL: Chemotherapy with/without osimertinib in patients with EGFRmadvanced NSCLC and progression on first-line osimertinib. Presented at: IASLC 2021 World Conference on Lung Cancer; September 8-14, 2021; virtual. Abstract P47.11.
