Lunning Discusses Defining and Approaching Treatment for Early-Relapsed DLBCL
During a Targeted Oncology™ Case-Based Roundtable™ event, Matthew A. Lunning, DO, and participants discussed how they approach treatment for a patient with diffuse large B-cell lymphoma who relapsed 8 months after primary therapy.
Matthew A. Lunning, DO (MODERATOR)
Assistant Vice Chancellor of Clinical Research
Associate Vice Chair of Research Department of Internal Medicine
Associate Professor, Division of Oncology and Hematology
University of Nebraska Medical Center
Omaha, NE

EVENT REGION Minnesota and Nebraska
PARTICIPANT LIST Dahlia H. Elkadi, MBBCh | Kurt Demel, MD | Kartik Anand, MD | Peter Silberstein, MD | Priya Kumar, MD | Birendra Kumar, MD | Pavan K. Bhamidipati, MBBS
CASE SUMMARY
A 67-year-old man presented with fatigue, back pain, and lymphadenopathy. He had a medical history of hypertension that was well controlled with medication. On physical examination, he had a 1.5-cm left posterior cervical node, 2.5-cm right anterior cervical node, and 2.0-cm left supraclavicular node. A PET/CT scan showed multiple enlarged mesenteric and retroperitoneal nodes, the largest measuring 5.3 × 3.1 cm. A bone marrow biopsy was negative. He received a diagnosis of diffuse large B-cell lymphoma (DLBCL), non–germinal center B-cell (GCB), double-expressor lymphoma.
Immunohistochemistry was positive for CD20, BCL6, BCL2 (50% of cells), MYC (> 30% of cells), Ki-67 85%, and MUM1 and negative for CD10. Complete blood count was normal, and lactate dehydrogenase level was elevated. Disease staging was stage III, high-intermediate risk, non-GCB, and he had an ECOG performance status of 1. Fluorescence in situ hybridization was negative for translocations involving MYC, BCL2, and BCL6.
R-CHOP (rituximab [Rituxan], cyclophosphamide, doxorubicin hydrochloride [hydroxydaunomycin], vincristine sulfate [Oncovin], and prednisone) was initiated for 6 cycles, and the back pain resolved. Posttreatment PET demonstrated a complete response with a Deauville score of 2; the patient was observed. Eight months after completion of therapy, he complained of fever, night sweats, and back pain. A palpable lymph node in the left groin was discovered on physical examination. A PET/CT scan showed a new left inguinal lymph node, an increase in size of a residual node, and multiple metabolically active lesions in lymph nodes of the retroperitoneum, abdomen, and pelvis. A biopsy confirmed DLBCL; next-generation sequencing was not performed.
DISCUSSION QUESTIONS
- How do you define primary refractory disease in DLBCL?
- How do you define early relapse?
- What is the prognosis for a patient like this?
- What therapeutic options would you consider for this patient?
LUNNING: Dr Elkadi, how do you define primary refractory disease in your practice?
ELKADI: I would say [it is if] they did not respond to the first chemotherapy regimen or did not get a complete response. This is how I would define primary refractory disease. Early relapse, less than 12 months.
LUNNING: And in St Cloud, are you getting Deauville scores from your nuclear medicine physicians for your end-of-treatment responses?
ELKADI: Yes, and I tend to get a PET scan after 2 cycles.
LUNNING: In Minneapolis, are you able to get Deauville scores from your nuclear medicine radiologists? And do you agree or have any different definitions of primary refractory or early relapse in DLBCL?
DEMEL: I would agree with the prior comments that nuclear medicine [radiologists] are giving us good readings after 2 cycles, and we’re getting a Deauville score, and we’re seeing interval response based on initial chemotherapy choices.
LUNNING: Do you have an interim time point of interest? Prior to cycle 3 or prior to cycle 5?
DEMEL: I also do a PET scan after 2 cycles.
LUNNING: Very good. Dr Anand, what is your prognosis for a patient like this, somebody who relapses within a year from the end of therapy?
ANAND: I think that’s where the CAR [chimeric antigen receptor] T-cell therapy plays a role. And the prognosis, [he has] non-GCB, which is a big subclass, has the ABC [activated B-cell type] and other categories, based on how we know gene expression profiling in these patients. Those are the patients who have early relapse, in my mind. In the front line, 60% to 70% are cured, and then 30% relapse. And for those who relapse, the 2-year survival is very low.
LUNNING: Yes, we’ve learned from the old chemotherapy days that for those patients who have primary refractory disease, median overall survival is about 6 to 7 months, based upon the SCHOLAR-1 data.1 Even when we’ve seen more modern data in the PET/CT era, we’re struggling in this population, even when we look into some of the randomized second-line trials. Dr Silberstein, what therapeutic options would you consider for this patient?
SILBERSTEIN: The first option I would think is CAR T-cell therapy. Hopefully it’s available for the patient, but that’s far and away the best treatment option.
LUNNING: Dr Priya Kumar, what are your thoughts on certain therapeutic options? Is CAR T-cell therapy your go-to in Minneapolis?
P. KUMAR: Yes. We have the University of Minnesota. We have Mayo Clinic. We certainly work closely with colleagues in both institutions, so we are lucky in that respect. This patient is 67 years old, [and] he’s relatively young. The overall prognosis for primary refractory disease is poor. I would go for the best treatment available in this setting and see whether he’s eligible for CAR T-cell therapy. He doesn’t seem to have any major organ issues.
I don’t think he has anything major that stands out as not being eligible for CAR T-cell therapy, so I would send him for a discussion. And if he’s CAR T cell ineligible, [I would] consider the other options that we have. I’ve used polatuzumab [vedotin-piiq (Polivy)]/bendamustine [Bendeka]/rituximab in that setting, [and] you could look at other options as well.
LUNNING: Dr Birendra Kumar, all the way from Arlington, Minnesota. How about in this situation, [what are] your thoughts on CAR T-cell therapy and your nearest treatment center for CAR T-cell [therapy] or transplant?
B. KUMAR: The 2 places that do all these transplants and CAR T-cell therapy are the University of Minnesota and Mayo Clinic, Rochester, and I’m affiliated with the University of Minnesota, so that’s my first choice. The good thing is the same people do both the autologous stem cell transplant [ASCT] and the CAR T-cell therapy, so I usually call them and ask them to evaluate. This patient, 9 months from the algorithm, early relapse, would be considered first for CAR T-cell therapy. But I don’t see why ASCT can’t be done, and CAR T-cell therapy can be done later also.
LUNNING: Yes, in some of these discussions…as we debate this topic, you can do a CAR T-cell therapy after ASCT, but it’s very hard to do ASCT after CAR T-cell therapy.
DISCUSSION QUESTION
- What is your reaction to the safety data for combination tafasitamab-cxix (Monjuvi)/lenalidomide (Revlimid)?2
LUNNING: Dr Bhamidipati, what are your thoughts about the safety profile of tafasitamab/lenalidomide?
BHAMIDIPATI: The cytopenias are not that bad. When I see the long-term [adverse] events of lenalidomide, [they are] very much manageable…with the dose adjustments. Seeing that many patients are on long-term tafasitamab—almost like 5 years—makes this a much more doable treatment, except for the front-loading, than polatuzumab with rituximab/bendamustine. Also, I have some trouble with giving polatuzumab and rituximab for a long term together [because of] the immunosuppression, so I think it’s not that bad, even with the infection risk. Tafasitamab with lenalidomide is probably better with less infection.
LUNNING: Have you had experience with tafasitamab/lenalidomide?
BHAMIDIPATI: Just 1 patient. My patient was [started] a few months ago, and I modified the treatment. I did not do the day 1 and 4 loading [dose of tafasitamab].2 [For lenalidomide,] I did more like 3 weeks on, 1 week off for the first 3 months and then changed it to every other week, mostly for cytopenias. This [combination] was used in a third-line patient, not in a second-line patient.
LUNNING: Dr Kumar, what are your thoughts on the safety profile of tafasitamab/lenalidomide? Do you believe the AEs [adverse effects] are driven by the lenalidomide more so than the tafasitamab?
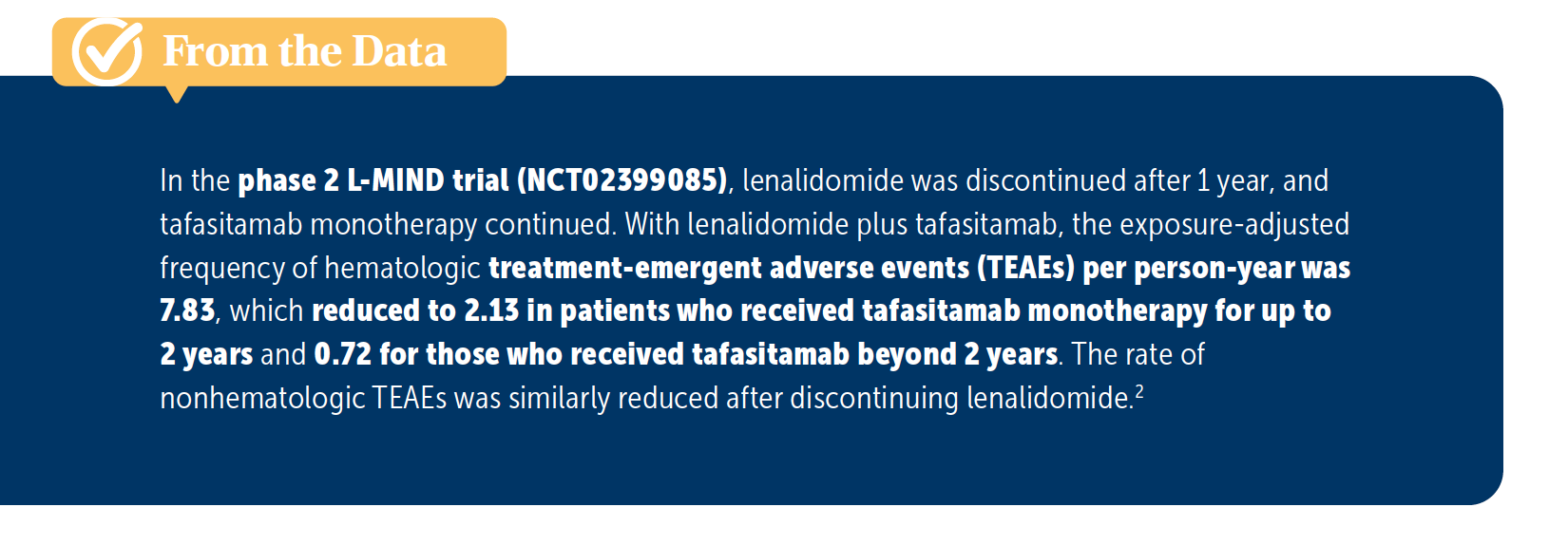
P. KUMAR: The lenalidomide is my guess because I’ve had experience with lenalidomide and there are cytopenias, especially in the older patients. They need frequent dose reductions. The data beyond 2 years [were] with tafasitamab alone, [correct]?
LUNNING: Yes. The lenalidomide stops after 1 year.
P. KUMAR: Yes, and the cytopenias are much less, [based on those data]. I think they are from lenalidomide, but I have no experience with the regimen.
LUNNING: Yes, it seems like in the year 1 to 2, there could be some cytopenia hangover from the lenalidomide, but it appears to improve and lessen over time [From the Data2]. Dr Anand, what strikes you about the safety data with tafasitamab/lenalidomide?
ANAND: I [don’t] think…we saw [anything too surprising]. Everybody in the community has experience with lenalidomide, and everybody can manage the diarrhea. More so, we know for patients who cannot digest milk, who are lactose intolerant, not to start lenalidomide [and] those kinds of [details]. But I didn’t see anything that is out of the ordinary in the [phase 2] study [L-MIND; NCT02399085]. In my experience…this regimen is very well tolerated, and it mirrors [the needs of] many of the patients we see in the Midwest who are farmers or otherwise who don’t want to do CAR T-cell therapy.
REFERENCES
1. Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800-1808. doi:10.1182/blood-2017-03-769620
2. Duell J, Abrisqueta P, Andre M, et al. Five-year efficacy and safety of tafasitamab in patients with relapsed or refractory DLBCL: final results from the phase II L-MIND study. Cancer Res. 2023;83(suppl 8):CT022. doi:10.1158/1538-7445.AM2023-CT022