Longer Follow-Up Shows Improved DFS With Nivolumab in MIUC
According to Matthew Galsky, MD, results from the CheckMate-274 clinical trial support adjuvant nivolumab as a standard of care for patients with high-risk muscle-invasive urothelial cancer after radical resection.
Matthew Galsky, MD

Treatment with the immune checkpoint inhibitor nivolumab (Opdivo) continued to induce improved disease-free survival (DFS) among patients with high-risk muscle-invasive urothelial carcinoma (MIUC) following radical surgery, regardless of previous treatment with neoadjuvant chemotherapy, nodal involvement, or PD-L1 status, according to extended follow-up from the phase 3 CheckMate 274 trial (NCT02632409) presented at the 22nd Annual Meeting of the Society of Urologic Oncology in December 2021.1
“With longer follow-up, nivolumab continued to demonstrate a clinically meaningful improvement in disease-free survival vs placebo for patients with high-risk muscle-invasive urothelial cancer after radical surgery. This disease-free survival benefit was observed in both the intent-to-treat [ITT] population and in the subset of patients with tumors harboring high levels of PD-L1 expression; a disease-free survival benefit with nivolumab was observed in most prespecified clinical subgroups,” Matthew Galsky, MD, professor of medicine (hematology and medical oncology), director of genitourinary medical oncology, codirector of the Center of Excellence for Bladder Cancer at The Tisch Cancer Institute; and associate director for translational research at The Tisch Cancer Institute at Mount Sinai in New York, New York, said during a virtual presentation of the poster.
The randomized, double-blind, multicenter CheckMate 274 trial enrolled 709 patients with high-risk MIUC originating in the bladder, ureter, or renal pelvis, including 353 in the ITT nivolumab group (PD-L1 ≥ 1%, n=140) and 356 in the placebo group (PD-L1 ≥ 1%, n=142).2
Patients were randomized 1:1 and stratified based on PD-L1 expression, pathological nodal status, and treatment with a neoadjuvant cisplatin-based chemotherapy. A dose of 240 mg of nivolumab or placebo was administered every 2 weeks intravenously for up to 1 year or until disease recurrence or trial discontinuation.
The primary end point of the study was DFS for all patients in the ITT population, as well as those with a PD-L1 expression of 1% or greater. The key secondary end point was non–urothelial tract recurrence-free survival (NUTRFS) in the ITT population and in patients with PD-L1 expression of 1% or greater. Moreover, distant metastasis-free survival (DMFS) was an exploratory end point. DFS was also evaluated in prespecified subgroups.
To be eligible for the trial, the high-risk population of patients with MIUC who had previously undergone radical surgery needed to have undergone radical surgery with or without cisplatinbased chemotherapy within 120 days prior to randomization. They also were required to have an ECOG performance status of 0 or 1.
Following a previous minimum follow-up in the ITT population of 5.9 months, DFS was significantly improved with nivolumab, compared with placebo, both in the ITT population (HR, 0.70; 98.22% CI, 0.55-0.90; P< .001) and in the population with tumor PD-L1 expression of 1% or greater (HR, 0.55; 98.72% CI, 0.35-0.85; P< .001). The safety profile of nivolumab was consistent with that observed in previous trials.
Following an additional follow-up of 5 months, which included a minimum follow-up in the intent-to-treat (ITT) population of 11 months (median follow-up in the nivolumab vs placebo group, 24.4 months vs 22.5 months, respectively) and 11.4 months in the PD-L1 of 1% or greater group (median follow-up in the nivolumab vs placebo group, 25.5 months vs 22.4 months), DFS benefit with nivolumab was maintained.
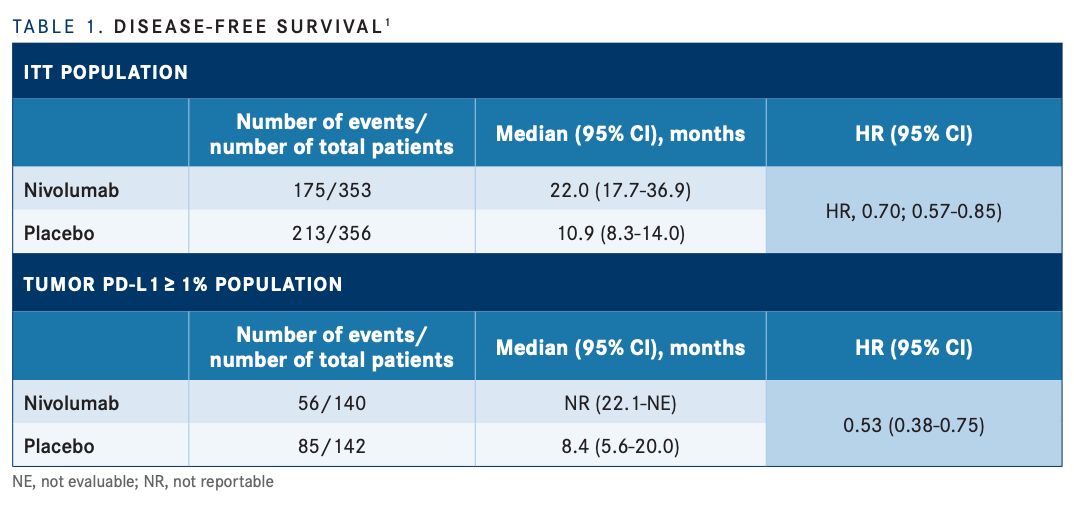
With the longer follow-up, the anti–PD-1 antibody demonstrated improved DFS, compared with placebo, in the ITT nivolumab and placebo groups (22.0 months vs 10.9 months, respectively), as well as in the PD-L1 of 1% or greater groups (not reached [NR] vs 8.4 months; TABLE 11 ). DFS probability at 12 months was 63.5% with nivolumab and 46.9% with placebo in ITT patients, and 67.6% vs 46.3%, respectively, among patients with PD-L1 expression of 1% or greater.
In the subgroup analysis, improved DFS was observed with nivolumab, compared with placebo, for those in subgroups including age (<65: HR, 0.73; 95% CI, 0.53-1.01), sex (female: HR, 0.77, 95% CI, 0.50-1.17), ECOG performance status (1: HR, 0.79; 95% CI, 0.56-1.12), nodal status (N0/x with <10 nodes removed: HR, 0.85; 95% CI, 0.57-1.27), use of prior cisplatin-based chemotherapy (No: HR, 0.90; 95% CI, 0.68-1.18), and PD-L1 status (<1%: HR, 0.82; 95% CI, 0.63-1.05).
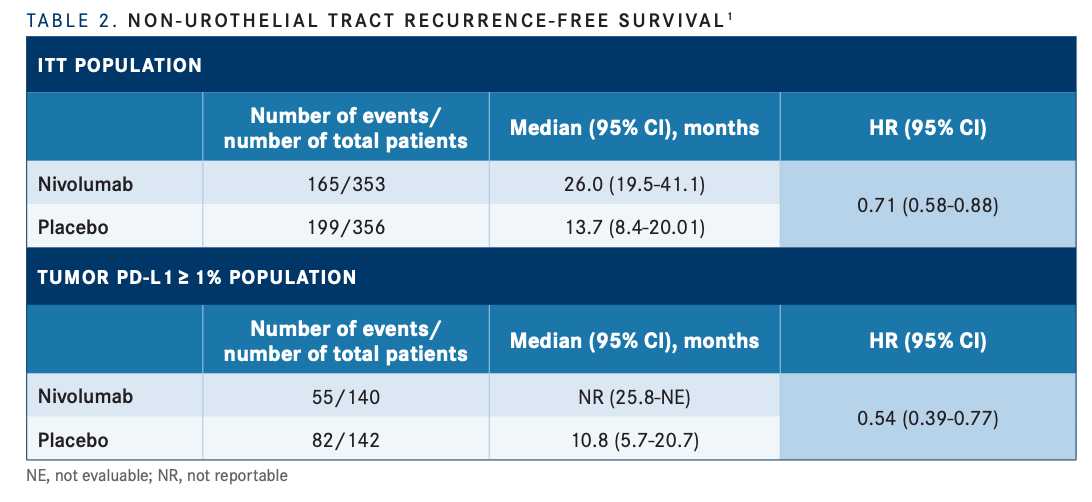
At 12 months, NUTRFS probabilities were also improved with nivolumab, vs placebo, in both the ITT (65.8% vs 50.6%, respectively) and PD-L1 expression of 1% or greater (69.2% vs 47.1%) populations. The median NUTRFS in the ITT was 26.0 months (95% CI, 19.5-41.1) vs 13.7 months (95% CI, 8.4- 20.0) (HR, 0.71; 95% CI, 0.58-0.88), respectively (TABLE 21 ).
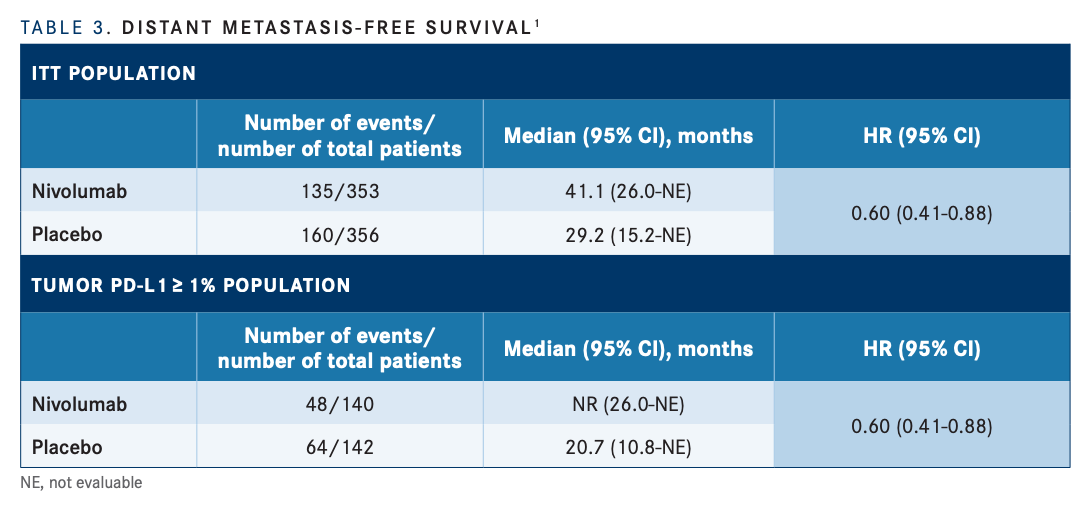
Similarly, DMFS was also improved with nivolumab, compared with placebo, in both the ITT (41.1 months [95% CI, 26.0-NE] vs 29.3 months [95% CI, 15.2-NE], respectively; HR, 0.73; 95% CI, 0.58-0.92) and PD-L1 expression of 1% or greater groups (NR [95% CI, 26.0-NE] vs 20.7 months [95% CI, 10.8- NE]; HR, 0.60; 95% CI, 0.41-0.88; TABLE 31 ).
In terms of time to response, the medians among the nivolumab and placebo groups, respectively, were 25.8 months (95% CI, 19.6-NE) and 11.1 months (95% CI, 8.3-19.4) in the ITT population, and NR (95% CI, 25.8- NE) and 10.9 months (95% CI, 5.8-22.2) in those with PD-L1 expression of 1% or greater. Galsky added that subsequent systemic therapy was received by 30% of patients on the nivolumab arm and 37.6% of patients on the placebo arm.
Galsky added that subsequent systemic therapy was received by 30% of patients on the nivolumab arm and 37.6% of patients on the placebo arm.
The median duration of therapy was 10.1 months (range, 0-12.5) in the nivolumab group, compared with 8.6 months (range, 0-12.6) in the placebo group.
“These results support adjuvant nivolumab as a standard of care for patients with high-risk muscle-invasive urothelial cancer after radical resection,” Galsky said.
REFERENCES
1. Galsky MD, Witjes JA, Gschwend JE, et al. Disease-free survival with longer follow-up from the phase 3 CheckMate 274 trial of adjuvant nivolumab in patients who underwent surgery for high-risk muscle-invasive urothelial carcinoma. Presented at: 22nd Annual Meeting of the Society of Urologic Oncology; December 1-3, 2021; Orlando, FL. Poster 175.
2. Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021;384(22):2102-2114. doi:10.1056/NEJMoa2034442
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More