Komrokji Analyzes Strategies to Address Anemia With Ruxolitinib in Myelofibrosis
During a Targeted Oncology™ Case-Based Roundtable™ event, Rami Komrokji, MD, discussed issues related to dosing and management of patients receiving ruxolitinib for myelofibrosis.
Rami Komrokji, MD
Vice Chair, Malignant Hematology Department
Head of the Leukemia and MDS Section
Moffitt Cancer Center
Tampa, FL

CASE SUMMARY
- A 68-year-old woman presented to her physician with symptoms of mild fatigue, moderate night sweats, and abdominal pain/fullness lasting 4 months; she also reported increased bruising and an unexplained 12-lb weight loss.
- Her spleen was palpable 8 cm below the left costal margin.
- Karyotype: 46,XX
- Bone marrow biopsy results: megakaryocyte proliferation and atypia with evidence of reticulin fibrosis
- Genetic testing results: JAK2 V617F mutation; CALR mutation negative
- A blood smear result revealed leukoerythroblastosis.
- Laboratory values
- Red blood cell count: 3.40 × 1012/L
- Hemoglobin level: 13.2 g/dL
- Hematocrit: 36%
- Mean corpuscular volume: 94 fL
- White blood cell count: 23.0 × 109/L
- Platelet count: 450 × 109/L
- Peripheral blood blasts: 1%
- Diagnosis: primary myelofibrosis
- Risk
- International Prognostic Scoring System: intermediate-2
- Mutation and Karyotype-Enhanced International Prognostic Scoring System for Primary Myelofibrosis in adults 70 years or younger: intermediate
- The patient refused stem cell transplant and received ruxolitinib (Jakafi).
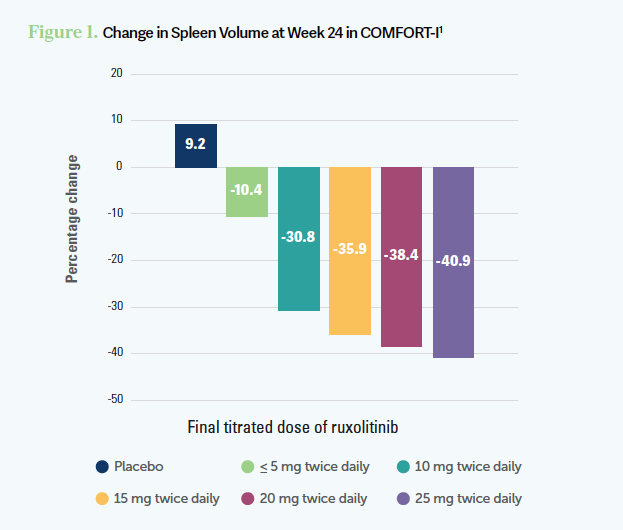
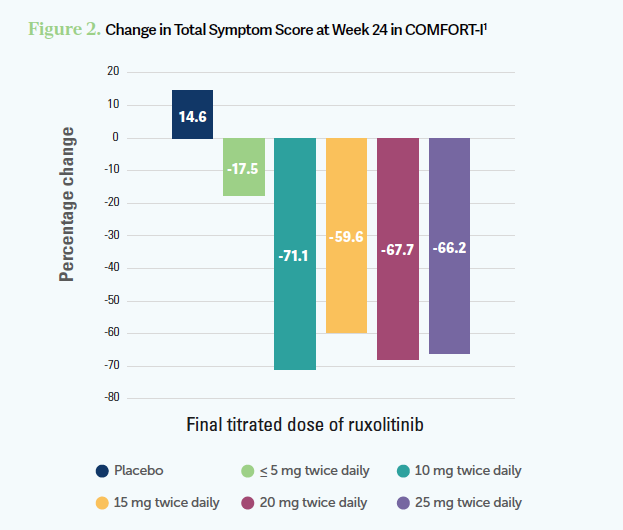
Targeted Oncology: What effect did the titrated dose of ruxolitinib have on spleen volume and total symptom score in the COMFORT-I trial (NCT00952289)?
KOMROKJI: The spleen volume [reduction] is dose dependent [Figure 11]. You will rarely see a meaningful spleen reduction at below 10 mg twice a day, and it takes 3 to 4 months. That becomes the challenge. For the patients who [have] borderline [cytopenia], the more you’re going to push those [patients] to get the spleen response, the more they are going to [develop cytopenia]. The symptom improvement can be seen daily at 5 mg or 10 mg twice a day and [comes] faster. You could achieve that with a lower [dosage] than the spleen response [Figure 21]. Ruxolitinib is typically given twice a day because it has a short half-life. Sometimes in real-life practice, especially if the patients [were prescribed] a certain [dosage] of the pill, until we get the other [dosage of the] pill, we sometimes do it once a day or we tell them to cut the pill and do it twice a day. But ideally, it should be done twice daily.
What hematologic toxicities were observed with ruxolitinib in the COMFORT-I and COMFORT-II (NCT00934544) trials?
This drug, in general, is safe. The major issues with ruxolitinib are anemia, thrombocytopenia, and less often neutropenia, and those are very predictable, particularly the anemia. It’s almost a 2 g/dL hemoglobin [level] drop by week 8. If you started somebody with hemoglobin [level] of 13 g/dL…[by] month 2, it will be 11 g/dL. But for many of those patients, their symptoms are better and their spleen [enlargement] is gone, so they don’t mind the 11-g/dL [hemoglobin level]. If somebody started with hemoglobin [level] of 9 g/dL or 8 g/dL and [it is] dropping to 7 g/dL, then they will probably need transfusions.
The only way to mitigate the cytopenia most of the time is dose reductions. Sometimes for anemia, we add ESAs [erythropoiesis-stimulating agents]. The COMFORT-I trial did not allow adding any ESAs. The COMFORT-II trial from Europe had a small subset who had ESA addition.2 Technically, ruxolitinib inhibits the JAK/STAT pathway where erythropoietin signals through, but because it has a short half-life, it’s a reversible inhibition. There is a window of time where the ESA can work. Sometimes we do that in practice to mitigate anemia.
There is no difference in the response based on [grade 3 or 4] anemia, so whether the patients [had anemia] or not, in terms of their spleen and symptoms, they improved.3 Sometimes we used to push the dose before having the newer JAK2 [Janus kinase 2] inhibitors, and I used to struggle with this. The University of Texas MD Anderson Cancer Center [in Houston] had a philosophy of starting the patients on the higher dose even if they [have] transfusion [dependence]; then you back off. We escalated the dosing a little more, but at least the spleen and symptom improvement, even in the setting of anemia, can be achievable. I struggle with rendering somebody [with] transfusion [dependence] especially now that we have other choices.
What else should physicians keep in mind when using ruxolitinib?
If someone gets admitted for pneumonia or surgery, especially if they are on higher dosing and [are] responding, those drugs cannot be stopped cold turkey, particularly ruxolitinib and probably fedratinib [Inrebic] as well, because patients will have rebound cytokines. And if they are sick with pneumonia, they will [risk experiencing] SIRS [systemic inflammatory response syndrome] or septic shock. Even if they are going to surgery, we try not to interrupt if they can take [medication] orally. If your patients are not able to take oral medications, sometimes we bridge them with steroids as they are admitted.
If the case patient’s baseline hemoglobin level of 13 g/dL reduced to below 8 g/dL, is that a sign of ruxolitinib failure or progression?
That’s not typical, but I’ve seen some patients who will get profound cytopenia [later], so whether it’s a red flag or just sensitivity, I don’t know. Sometimes it depends on the timing. If somebody was on ruxolitinib for...a couple of years and I see this anemia, that’s…a red flag [ for] if there is anything changing the disease. If it was the first few months of ruxolitinib, I would assume it’s more likely to be ruxolitinib related. I would try to back off the dosing, [do] the classical things in medicine, make sure that the patient does not bleed [in case] something else was going on. But it will probably be somebody who is more sensitive. With that degree of drop, if I cannot get a spleen response or constitution symptoms relieved with a lower dose than nowadays, sometimes we would consider shifting to other JAK2 inhibitors if it was ruxolitinib related and I could not manage the symptoms with a lower dose.
How do you address the increased risk of skin cancer with ruxolitinib?
There are not very conclusive data, but there are data all over the place that any patients [with myelofibrosis] are at higher risk of skin cancer. For both hydroxyurea [Hydrea] and ruxolitinib, [it’s] a little higher than in patients who were not on treatment.4,5 Practicing in Florida adds another risk factor [for our patients developing skin cancer]. We tell them to get a baseline [dermatology exam] when we are starting and then get surveillance every 6 months. We don’t have many alternatives for treatment because both hydroxyurea and ruxolitinib increase the risk. There is no literature to say it’s dose dependent. I’ve rarely stopped the treatment because most of the time, if you’re doing surveillance, you can detect those early, but I’ve had patients who had bad...squamous cell carcinoma that [required] resection…. I held the treatment and tapered down, not knowing what else to do, because it…was extensive. There is literature, but there is no guidance.
What is the lowest dose of ruxolitinib you would use before making a switch?
The dosing of ruxolitinib is dependent on what I’m trying to achieve. If I’m going for a spleen response, I want them at least to be on 10-mg dosing because I rarely see a spleen response below 10 mg twice daily. [With] symptom improvement and [if] they’re feeling better [with] 5 mg to 10 mg, I settle and may not push the dosing more.
How do you safely switch from ruxolitinib to pacritinib (Vonjo)?
In terms of shifting, there is not much guidance, but we always taper down the ruxolitinib. We don’t stop it. If we’re shifting to pacritinib, [physicians] do different things. We taper down the ruxolitinib and sometimes put them on a bridge of steroids. I’ve had some colleagues who overlapped them in the first couple of weeks. Pacritinib has a much longer half-life, so it takes longer to kick in.6 With ruxolitinib, you see symptom control in a week or 2; with pacritinib, it takes 3 to 4 weeks. The approach is not to stop immediately.... Tapering ruxolitinib is 100% agreed on, particularly if you are stopping because of cytopenias, [but if] the patients were responding, that rebound will be even worse than [for] somebody who’s [experiencing progression]. The alternative strategies are to put them on a steroid bridge or overlap them…for a week or two and then stop the ruxolitinib completely. There is not much guidance if somebody was on pacritinib if you’re bridging them to something else, [and the same applies] with fedratinib. Pacritinib has less or no JAK1 effect, so in my experience, stopping it immediately is more tolerable. You don’t have to taper it. But we do with fedratinib. There are not many data yet with momelotinib [Ojjaara].
For patients who do not respond to management of anemia and require transfusions every 2 weeks, would you be concerned about iron overload?
There is not much literature about iron overloading in myelofibrosis, but it’s [a] very similar story to MDS [myelodysplastic syndrome]. Myelofibrosis is an inflammatory condition, so patients could have a high baseline [level] of ferritin. To [identify] somebody [having] iron overload, you have to get to the threshold of 15 to 20 units of blood transfusions.
The responses to ESA are [fewer] than in MDS because those patients have a higher endogenous serum erythropoietin level; you rarely see responses. There have been studies looking at…combination ruxolitinib/danazol and ruxolitinib/thalidomide…with modest activity.7,8 There is an ongoing phase 3 trial [INDEPENDENCE; NCT04717414] looking at ruxolitinib vs ruxolitinib/luspatercept-aamt [Reblozyl] because in the phase 2 trial [ACE-536-MF-001; NCT03194542], it seemed…that luspatercept can work in myelofibrosis to help with ruxolitinib-induced anemia.9 If patients have lower-risk myelofibrosis or I estimate their survival to be in years and they [have] high transfusion burden, I may consider some iron chelation. The problem [is] that the decision has to be individualized because even in MDS, we do the same because those iron chelations come with their own adverse events and toxicities. The subcutaneous pump is not easy for patients, and the oral ones have renal insufficiency, etc. But in selected cases, you could consider it.
If a patient with baseline hemoglobin level of 8 g/dL receives ruxolitinib and develops transfusion dependence, at what point do you consider it to be ruxolitinib failure?
[With] momelotinib approved, it will probably start taking that area of patients who [have anemia] at baseline because momelotinib does have anemia response in almost 25% of patients.10 I would say [to wait] 3 to 4 months for the main thing you are [managing]. If you’re [managing] the spleen and constitutional symptoms [and] you don’t see the spleen response by 3 to 4 months, then that’s failure. The [time to consider it] intolerance to treatment if they develop profound cytopenias is 2 to 3 months as well. I look at…am I achieving the primary end point? Did I at least reduce the spleen [size] by 25%? Is the patient feeling better at 4 months? If yes, I would continue. The other part I look at is if I make the patient [have] transfusion [dependence], are they having severe cytopenias? Sometimes you can start making the call on that at 2 months.
What approaches to controlling anemia related to myelofibrosis are most promising?
Whatever you use for anemia, [approximately] 30% response is the best [we’ve achieved]. In my experience of patients who [have cytopenia] without any spleen or constitutional symptoms, the most success had been with thalidomide plus prednisone, [where] 1 of 4 patients would respond. But if there was a response, some of the responses were very durable. You do the steroids the first 3 months, you take them off, [and] then you continue to thalidomide. I’ve had patients on [JAK inhibition] for 7 or 8 years. With the newer JAK2 inhibitors, pacritinib and momelotinib, the story is changing a little bit, particularly if they have spleen and constitutional symptoms. Then you are [managing] both. We still have to see data on somebody who [has cytopenia] without any spleen or constitutional symptoms [and] how much they will benefit from something like momelotinib or pacritinib for anemia.
REFERENCES
1. Verstovsek S, Gotlib J, Gupta V, et al. Management of cytopenias in patients with myelofibrosis treated with ruxolitinib and effect of dose modifications on efficacy outcomes. Onco Targets Ther. 2013;7:13-21. doi:10.2147/OTT.S53348
2. McMullin MF, Harrison CN, Niederwieser D, et al. The use of erythropoiesis-stimulating agents with ruxolitinib in patients with myelofibrosis in COMFORT-II: an open-label, phase 3 study assessing efficacy and safety of ruxolitinib versus best available therapy in the treatment of myelofibrosis. Exp Hematol Oncol. 2015;4:26. doi:10.1186/s40164-015-0021-2
3. Verstovsek S, Mesa RA, Gotlib J, et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366(9):799-807. doi:10.1056/NEJMoa1110557
4. Lin JQ, Li SQ, Li S, et al. A 10-year retrospective cohort study of ruxolitinib and association with nonmelanoma skin cancer in patients with polycythemia vera and myelofibrosis. J Am Acad Dermatol. 2022;86(2):339-344. doi:10.1016/j.jaad.2021.10.004
5. Hydrea. Prescribing information. Bristol Myers Squibb; 2021. Accessed November 20, 2023. https://tinyurl.com/dt9v7jft
6. Mascarenhas J, Hoffman R, Talpaz M, et al. Pacritinib vs best available therapy, including ruxolitinib, in patients with myelofibrosis: a randomized clinical trial. JAMA Oncol. 2018;4(5):652-659. doi:10.1001/jamaoncol.2017.5818
7. Gowin K, Kosiorek H, Dueck A, et al. Multicenter phase 2 study of combination therapy with ruxolitinib and danazol in patients with myelofibrosis. Leuk Res. 2017;60:31-35. doi:10.1016/j.leukres.2017.06.005
8. Duan M, Zhou D. Improvement of the hematologic toxicities of ruxolitinib in patients with MPN-associated myelofibrosis using a combination of thalidomide, stanozolol and prednisone. Hematology. 2019;24(1):516-520. doi:10.1080/16078454.2019.1631509
9. Gerds AT, Harrison C, Kiladjian JJ, et al. Safety and efficacy of luspatercept for the treatment of anemia in patients with myelofibrosis: results from the ACE-536-MF-001 study. J Clin Oncol. 2023;41(suppl 16):7016. doi:10.1200/JCO.2023.41.16_suppl.7016
10. Gerds AT, Verstovsek S, Vannucchi AM, et al. Momelotinib versus danazol in symptomatic patients with anaemia and myelofibrosis previously treated with a JAK inhibitor (MOMENTUM): an updated analysis of an international, double-blind, randomised phase 3 study. Lancet Haematol. 2023;10(9):e735-e746. doi:10.1016/S2352-3026(23)00174-6

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More