Integrating Immunotherapy With Chemoradiation in the Treatment of Head and Neck Cancer
Squamous cell carcinoma of the head and neck (SCCHN) is an immunosuppressive disease, as its patients often demonstrate immune dysregulation that correlates with poorer outcomes. Trials are underway combining chemoradiotherapy with immune checkpoint inhibitors.
Abstract
Squamous cell carcinoma of the head and neck (SCCHN) is an immunosuppressive disease, as its patients often demonstrate immune dysregulation that correlates with poorer outcomes. Immune checkpoint inhibitors, virally-directed T-cell therapy and vaccines have been explored with early studies showing some benefit in recurrent, metastatic SCCHN. The response rate to available immune checkpoint therapies in patients with advanced SCCHN is estimated at 20% in an unselected population, and may be higher in those with programmed death ligand 1 (PD-L1)-positive tumors. Cetuximab is used in the definitive or metastatic setting and has demonstrated immune modulating effects that promote expression of immune checkpoint receptors. Platinum-based chemotherapy and radiotherapy have both been associated with enhanced immunologic effects that may predict synergism with immune checkpoint inhibitors. Trials are underway combining chemoradiotherapy with immune checkpoint inhibitors in intermediate and poor-risk patients with SCCHN, while a de-intensification strategy combining radiotherapy and/or cetuximab with immune checkpoint inhibitors could be rationalized in favorable risk, human papillomavirus (HPV)-associated SCCHN. Trials should incorporate correlative studies as we investigate predictive biomarker selection in an era of more personalized medicine. This remains an exciting time in immuno-oncology, as there is a developing role for immunotherapies in the management of SCCHN not only in advanced disease, but also in the definitive treatment setting.
Introduction
Squamous cell carcinoma of the head and neck (SCCHN) often presents with locoregionally advanced disease requiring multimodality, definitive therapy. Most often, definitive therapy includes surgery followed by radio-therapy and/or chemotherapy or concurrent chemoradiotherapy (CRT). While human papillomavirus (HPV)-associated oropharyngeal SCCHN offers an improved prognosis in patients without other risk factors, locoregional recurrence or the development of distant disease remains a significant issue in patients with more traditional risk factors (tobacco and alcohol use).1,2Five-year survival in patients with distant or metastatic disease at any site remains dismal.3
Platinum-based, palliative systemic therapy combined with the epidermal growth factor receptor (EGFR) inhibitor cetuximab results in a median overall survival (OS) of 10 months and represents the standard of care in the upfront palliative setting for fit patients.4In fact, cetuximab remains the only approved targeted therapy for patients with SCCHN since its approval in 2006. For the overwhelming majority of patients with advanced SCCHN, survival remains poor and there is an urgent need to investigate novel therapies.
In recent years, there has been significant interest in exploiting mechanisms by which cancer cells are recognized by the immune system. Further understanding of immune dysregulation and tumor immune evasion has led to the recent investigation of systemic immunotherapies in both solid organ and hematologic malignancies, including SCCHN. It has become evident that tumor progression is promoted by immune evasion and alteration of an effective immune response by cancer cells.5One facet of immune evasion involves regulation of immune checkpoint pathways, facilitated by ligand-receptor interactions, that serves to prevent an excessive inflammatory response.6These immune checkpoint pathways are also exploited by chronic viral infections, such as HPV. Several immune checkpoint inhibitors are now available and have been explored in various malignancies, including SCCHN.5While the focus of recent immunotherapy use has been in the advanced or metastatic setting, the interaction between chemotherapeutics, radiotherapy, and immunotherapy is of particular interest. Understanding the potential synergistic effects of combining these therapies will yield more rational clinical trial design and more importantly, may improve outcomes in SCCHN patients in the definitive treatment setting.
Immunotherapy in Head and Neck Cancer
SCCHN appears closely linked with immunosuppression, as its patients often demonstrate impaired immune cell function that correlates with poorer outcomes.7,8While the immune system requires recognition of foreign tumor antigens to promote an immune response, immune dysregulation in SCCHN tumor cells decreases immunogenicity. Some ways in which this can occur are through the development of T-cell tolerance to ongoing HPV infection, modulation of inflammatory and angiogenic cytokines, downregulation of human leukocyte antigen (HLA) class I and antigen-processing machinery, and the expression of immune checkpoint ligands or receptors to promote immune evasion.9-12
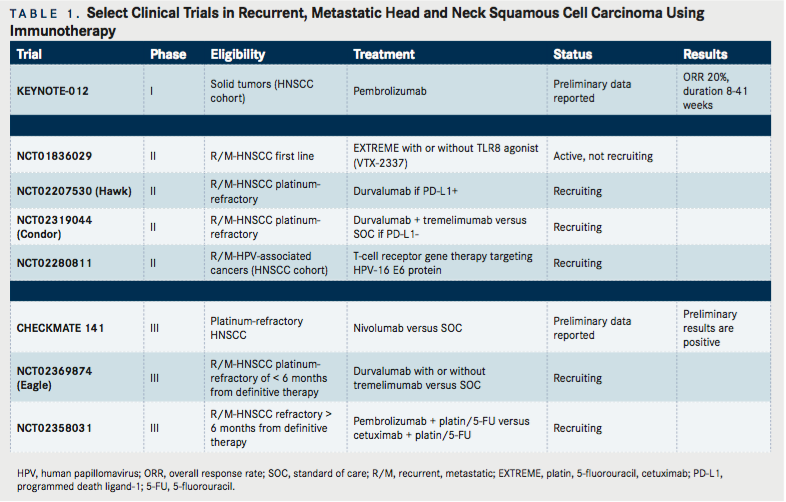
Immune checkpoint receptors serve to inhibit normal T-cell activation and co-stimulation to moderate a normal immune response.6Cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed death-1 (PD-1) are immune checkpoint receptors on the surface of immune cells (notably T-cells) that interact with their respective ligands on tumor cells (CTLA-4 with CD80, CD86; PD-1 with programmed death ligand 1 [PD-L1], PD-L2). High tumor expression of PD-L1 and/or PD-1 positive T-lymphocyte expression can promote T-cell dysfunction (or exhaustion) to favor tumor evasion.13,14Preclinical data has estimated PD-L1 expression at 60% in SCCHN tumors as measured by immunohistochemistry (IHC)15, although a clinically meaningful cutoff for PD-L1 positivity has yet to be determined. Similarly of interest, the surrounding immune infiltrate more often expresses PD-1 in HPV-positive as compared to HPV-negative tumorsdespite the improved prognosis of HPV-related disease—suggesting a complex relationship between anti-tumor immunity and inhibitory immune pathways.16Monoclonal antibodies against both CTLA-4 and PD-1 are actively being explored in a wide range of solid tumors since their clinical benefit was first established in metastatic melanoma.17In a phase I clinical trial investigating pembrolizumab (MK-3475, an anti-PD-1 antibody) in a heavily pre-treated, advanced SCCHN population overall response rate (ORR) was around 20%, and similar regardless of HPV status18(TABLE 1). We are awaiting the results of two phase III randomized trials in recurrent, metastatic SCCHN patients using nivolumab (anti-PD-1 antibody, NCT02105636) and pembrolizumab (NCT02358031), respectively. The use of anti-PD-L1 therapy (MEDI4736, durvalumab) has shown additional promise in a phase I/II trial with recurrent, metastatic SCCHN patients.19The ORR was 12%, but 25% in patients with PD-L1 positive tumors by IHC. Response duration ranged from 4 to 43 weeks, and median duration of response has not been reached. The toxicity profile of therapy was favorable with no adverse events resulting in discontinuation or death. This has prompted the development of a phase III study with durvalumab alone or in combination with a CTLA-4 inhibitor (tremelimumab) versus standard therapy in the first-line setting in recurrent, metastatic SCCHN patients (NCT02369874). Additional trials comparing immune checkpoint inhibitors with standard platinum-based therapy in the first-line metastatic setting are planned.
Despite interest in CTLA-4 and PD-1, there are multiple other immune checkpoint receptors that have modifying effects to promote tumor immune evasionnamely LAG-3, TIM-3, and killer-cell immunoglobulin-like receptors (KIRs). There are several ongoing clinical trials aiming to combine monoclonal antibodies targeting these receptors with anti-CTLA-4 and anti-PD-1 therapies. Similarly, therapies aimed at harboring immune stimulatory pathways, such as OX-40 and CD137, are enrolling and accruing SCCHN patients. Despite these advances, data from other solid tumors suggest that positive PD-L1 IHC staining does not always correlate with a response to immunotherapy.20Given that response to available immunotherapies in patients with advanced SCCHN is estimated at 20% in an unselected population, there is strong interest in identifying predictive biomarkers that may identify those likely to respond. Additionally, the promise of immunotherapy has sparked interest in moving these therapies to the definitive treatment setting, combining them with chemotherapy and radiotherapy.
In addition to immune checkpoint and stimulatory targeting, there have been significant efforts to utilize vaccine-based approaches that take advantage of HPV epitopes and T-cell mediated therapy against Epstein-Barr virus (EBV) in nasopharyngeal carcinoma that remain in early clinical development.21While these agents are not the focus of this review, one could envision virally targeted immune therapy in the pre-treatment setting or as maintenance therapy following definitive treatmentalthough these approaches have yet to be explored and more clinical data is needed to support their use.
Combining Cetuximab with Immunotherapy
Cetuximab is a monoclonal antibody therapy that targets the EGFR and represents the only targeted therapy approved for the management of SCCHN in the last 10 years. Cetuximab binds the EGFR on the surface of tumor cells allowing for the formation of EGFR-cetuximab immune complexes that are then recognized by tumor-specific cytotoxic T-cells following antigen presentation. These cytotoxic T-cells then mediate tumor cell elimination. Natural killer (NK) cell-dependent tumor cell lysis also plays an important role.22Despite this potential for cetuximab driven, immune-mediated cell death, several mechanisms may promote tumor immune evasion. T-regulatory cells (Tregs), that negatively regulate the normal immune response, can inhibit NK-cell and cytotoxic T-cell activities. In addition, a disruption in HLA and antigen-processing machinery can impair cytotoxic T-cell recognition. Understanding these immune modulating mechanisms is crucial, given that response rates to single-agent cetuximab in advanced SCCHN fail to exceed 15%.23
In a neoadjuvant, single agent study using cetuximab, Jie, et al, showed that following cetuximab treatment, there were increased intratumoral CTLA-4 expressing CD4+ Foxp3+ Tregs. Cetuximab expanded this Tregpopulationin vitro.24These increased Tregsmay suppress cetuximab-mediated antibody-dependent cellular cytotoxicity (ADCC). Usingex vivoassays, Jie and colleagues were able to show that ipilimumab (a CTLA-4 inhibitor) targeted this Tregpopulation and restored NK cell function.24Additionally, preclinical work in EGFR-mutated lung cancer has shown a correlation between EGFR pathway activation and a signature of immunosuppression. Immune checkpoint expression of PD-1/ L1 and CTLA-4 was upregulated along with multiple tumor-promoting cytokines thought to impair T-cell function.25
These data taken together support the use of cetuximab in combination with immune checkpoint inhibitors, and clinical trial planning is underway. A trial combining intensity-modulated radiotherapy (IMRT) with cetuximab and ipilimumab is currently accruing (NCT01860430) in patients with locoregionally advanced SCCHN. Given the difference in outcomes based on HPV status, some of these trials are incorporating platinum-based chemotherapy with anti-PD-1 therapy for HPV-negative patients and a chemotherapy-free approach in HPV-positive patients. Given the favorable outcomes in patients with HPV-associated disease, de-intensification strategies are appealing. Preclinical data supports the rationale for combining IMRT with cetuximab and/or immune checkpoint inhibition as a novel approach.
Combining Chemotherapy with Immunotherapy
While the global immunosuppressive effects of cytotoxic chemotherapy are appreciated, further investigation seeks to understand the more complex immune modulating effects of chemotherapy that may guide rational trial design using immunotherapy in combination. Of particular interest is the effect of platinum-based chemotherapy on tumoral immunity, given its pivotal role in the management of SCCHN.
Cisplatin works primarily as a cytotoxic agent to cross-link DNA and inhibit cellular mitosis, but recent work has established that it has additional immune modulating effects. Four main mechanisms of immune modulation have been suggested: (1) upregulation of MHC class I expression, (2) upregulation of cytotoxic T-cell activity, (3) proliferation of effector T-cells, and (4) downregulation of the immunosuppressive microenvironment.26MHC class I upregulation leading to more effective antigen recognition and activation of cytotoxic T-cells has been observed in SCCHN cell lines treated with platinum-based chemotherapy in addition to other solid tumors in murine models.27,28When a dose-dense, platinum-based chemotherapy combination was used in an ovarian cancer murine model, intratumoral CD8+ T-cell recruitment was enhanced, with anti-tumor effects.29When this was utilized in a small cohort of refractory ovarian cancer patients, levels of IL-2 and IFNγ increased in those with a treatment response, suggestive of cytotoxic T-cell activation.
Cisplatin-mediated immune effects in the context of immune checkpoint inhibitor use have recently been investigated in various solid tumor models. The addition of cisplatin with a CTLA-4 inhibitor resulted in increased intratumoral T-cell infiltration in a mesothelioma model, and the addition of anti-PD-1 therapy with CD137, a T-cell costimulatory molecule, led to increased anti-tumor effects across several cell types including lung epithelial cells transformed with HPV-16 E6 and E7 proteins.30,31In a nonsmall cell lung cancer population treated with adjuvant platinum-based chemotherapy, the CD4+ T-cell/Tregratio significantly increased, suggesting reduced immunosuppressive activity.32These findings all strengthen the rationale for combining cisplatin and immune checkpoint therapies, although preclinical modeling and clinical correlative studies in SCCHN are currently limited.
Early clinical studies combining chemotherapy with immunotherapy in advanced melanoma proved to be safe and feasible.33Ipilimumab has been used in combination with carboplatin and paclitaxel as first-line therapy for extensive small cell lung cancer in a phase II trial, which showed that a chemotherapy lead-in followed by combination chemotherapy and ipilimumab improved immune-related PFS by more than 1-month compared with chemotherapy alone.34Studies combining chemotherapy with immunotherapy in SCCHN are underway or being planned. A platinum-based chemotherapy regimen with cetuximab and a TLR-8 agonist is planned in the first-line metastatic setting (NCT01836029) and a phase III study combining a platinum-based regimen with either cetuximab or pembrolizumab in the recurrent, metastatic setting is recruiting (NCT02358031). Given the poorer outcomes of HPV-negative patients with locoregionally advanced disease, these data would support an intensification strategy that might combine IMRT, platinum-based chemotherapy, and an immune checkpoint inhibitor in the definitive setting (as discussed below).
Combining Radiotherapy with Immunotherapy
While the cytotoxic effects of radiotherapy are appreciated, increasing evidence has stimulated interest in understanding the immune effects of radiotherapy. Pre-clinical work has suggested that radiation exposure induces pro-inflammatory cytokines (IL-1β and TNFα)in vivothat may serve to enhance dendritic cell and T-cell activation.35,36Further studies have shown upregulation of death receptors (Fas/CD95), MHC class I, and costimulatory molecules in the setting of radiation, which may promote anti-tumor activity.37,38Radiotherapy has complex effects on the tumor microenvironment, such as homing of an immune infiltrate at the site of radiation exposure following inflammatory signaling. Early preclinical modeling has shown that the addition of the T-cell growth factor IL-2 and TNFα in combination with radiotherapy have shown anti-tumor effects.40,41
Attempts to combine CTLA-4 inhibitors with radiation in breast cancer models showed that the addition of an immune checkpoint agent induced a CD8+ T-cell mediated tumor response.42Murine breast cancer models also previously have demonstrated that following radiation treatment to a primary tumor, growth inhibition can occur in an outside field when combined with immunotherapythe abscopal effect.43This has been further documented in melanoma, where treatment with ipilimumab followed by radiation resulted in significant regression of disease at all sites, with further studies showing that CD4+ T-cell populations and tumor antigen targeting had increased post-radiation.44We have also observed a favorable trend for systemic response following palliative radiation in a series of melanoma patients treated with ipilimumab.45Of note, most of the preclinical studies and human case reports have observed synergistic benefit with relatively hypo-fractionated, short-couse radiation, although recently presented data regarding the immune effects of fractionated radiotherapy have also been reported. This involved assessing temporal changes in circulating T-effector cells, Tregs, and immune checkpoint co-expressing T-cells in a population of SCCHN patients treated with definitive therapy (unpublished data, Schoenfeld JD, et al.).
While phase I clinical trials using TNFα with radiation proved too toxic46, there remains preclinical rationale for combining immune checkpoint inhibitors with radiotherapy. Several studies are combining IMRT with CTLA-4 or PD-1 inhibitors in the definitive setting, either alone or in combination with radiation(TABLE 2). For example, RTOG 3504 will combine IMRT with cisplatin and nivolumab for HPV-negative patients with locoregionally advanced disease, and is in development. This study seeks to address the poorer outcomes of HPV-negative patients with locoregionally advanced disease by intensifying treatment with the addition of immunotherapy to a standard chemoradiation approach. Although building on a standard chemoradiation approach is naturally appealing, it is unknown whether the immunosuppressive effects of standard chemotherapy or approximately 7-weeks of fractionated radiation to both the primary tumor and nodal basin will impede anti-tumor immune responses.
An argument could also be made to use radiation and immune checkpoint therapy for patients with metastatic disease given immune modifying effects. Priming with immunotherapy followed by the use of focused irradiation can potentially allow for an abscopal effect at outside sites, but the optimal radiation and immunotherapy parameters to allow for this effect remain investigational.
Conclusions
This is an exciting time in immuno-oncology, and there is a developing role for immunotherapies in the management of SCCHN, not only in advanced disease, but in the definitive treatment setting. We illustrate how immune modulation can occur with biologic agents, chemotherapeutics, and radiotherapy, and how the addition of available immunotherapies may augment treatment responses. Correlative studies with tumor immune profiling will promote rational clinical trial design. We are hopeful that the benefits of immunotherapy in SCCHN are just being realized, given that the last novel agent approved in this disease was over a decade ago.
References
- Seiwert TY and Cohen EE. State-of-the-art management of locally advanced head and neck cancer.Br J Cancer. 2005;92(8):1341-1348.
- Marur S and Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis and treatment.Mayo Clin Proc. 2008;83(4);489-501.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975- 2012, NCI. Bethesda, MD, http://seer.cancer.gov/csr/1975_2012/
- Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer.N Engl J Med. 2008;359(11):1116-1127.
- Ferris RL. Immunology and immunotherapy of head and neck cancer.J Clin Oncol. 2015;33(29):3293-3304.
- Ramsay AG. Immune checkpoint blockade immunotherapy to activate anti-tumour T-cell immunity.Br J Haematol. 2013;162(3):313-325.
- Kuss I, Hathaway B, Ferris RL, et al. Decreased absolute counts of T lymphocyte subsets and their relation to disease in squamous cell carcinoma of the head and neck.Clin Cancer Res. 2004;10(11):3755-3762.
- Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome.Science. 2006;313(5795):1960-1964.
- O’Brien PM and Saveria Campo M. Evasion of host immunity directed by papillomavirus-encoded proteins.Virus Res. 2002;88(1-2):103-117.
- Stanley M. Immunobiology of HPV and HPV vaccines.Gynecol Oncol. 2008;109(2 Suppl):S15-21.
- Duffy SA, Taylor JM, Terrell JE, et al. Interleukin-6 predicts recurrence and survival among head and neck cancer patients.Cancer. 2008;113(4):750-757.
- The Cancer Genome Atlas Network: Comprehensive genomic characterization of head and neck squamous cell carcinomas.Nature. 2015;517(7536):576-582.
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade.Science. 1996;271(5256):1734-1736.
- Zandberg DP and Strome SE. The role of the PD-L1:PD-1 pathway in squamous cell carcinoma of the head and neck.Oral Oncol. 2014;50(7):627-632.
- Lyford-Pike S, Peng S, Young GD, et al. Evidence for a role of the PD-1:PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma.Cancer Res. 2013;73(6):1733-1741.
- Badoual C, Hans S, Merillon N, et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer.Cancer Res. 2013;73(1):128-138.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma.N Engl J Med. 2010;363(8):711-723.
- Seiwert TY, Burtness B, Weiss J, et al. A phase Ib study of MK-3475 in patients with human papillomavirus (HPV)-associated and non-HPV associated head and neck (H/N) cancer.J Clin Oncol. 2014;32:386s (abstr 6011).
- Segal NH. Safety and efficacy of MEDI4736, an anti-PD-L1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion.J Clin Oncol. 2015;33:150s (abstr 3011).
- Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (antiprogrammed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non–small-cell lung cancer.J Clin Oncol. 2015;33(18):2004-2012.
- Schoenfeld JD. Immunity in head and neck cancer.Cancer Immunol Res. 2015;3(1):12-17.
- Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape.J Clin Oncol. 2010;28(28):4390-4399.
- Vermorken JB, Trigo J, Hitt R, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy.J Clin Oncol. 2007;25(16):2171-2177.
- Jie HB, Schuler PJ, Lee SC, et al. CTLA-4 regulatory T cells increased in cetuximab-treated head and neck cancer patients, suppress NK cell cytotoxicity and correlate with poor prognosis.Cancer Res. 2015;75(11):2200-2210.
- Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors.Cancer Discov. 2013;3(12):1355- 1363.
- de Biasi AR, Villena-Vargas J, Adusumilli PS. Cisplatin-Induced Antitumor Immunomodulation: A Review of Preclinical and Clinical Evidence.Clin Cancer Res. 2014;20(21):5384-5391.
- Gelbard A, Garnett CT, Abrams SI, et al. Combination chemotherapy and radiation of human squamous cell carcinoma of the head and neck augments CTL-mediated lysis.Clin Cancer Res. 2006;12(6):18971905.
- Wan S, Pestka S, Jubin RG, et al. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells.PLoS ONE. 2012;7:e32542.
- Chang CL, Hsu YT, Wu CC, et al. Dose-dense chemotherapy improves mechanisms of antitumor immune response.Cancer Res. 2013;73(1):119127.
- Wu L, Yun Z, Tagawa T, et al. CTLA-4 blockade expands infiltrating T cells and inhibits cancer cell repopulation during the intervals of chemotherapy in murine mesothelioma.Mol Cancer Ther. 2012;11(8):1809-1819.
- Wei H, Zhao L, Li W, et al. Combinatorial PD-1 blockade and CD137 activation has therapeutic efficacy in murine cancer models and synergizes with cisplatin.PLoS ONE. 2013;8(12):e84927.
- Lee SY, Kang TH, Knoff J, et al. Intratumoral injection of therapeutic HPV vaccinia vaccine following cisplatin enhances HPV-specific antitumor effects.Cancer Immunol Immunother. 2013;62(7):1175-1185.
- Weber J, Hamid O, Amin A, et al. Randomized phase I pharmacokinetic study of ipilimumab with or without one of two different chemotherapy regimens in patients with untreated advanced melanoma.Cancer Immun. 2013;13:7.
- Reck M, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial.Ann Oncol. 2013;24(1):75-83.
- Ishihara H, Tsuneoka K, Dimchev AB, et al. Induction of the expression of the interleukin-1 beta gene in mouse spleen by ionizing radiation.Radiat Res. 1993;133(3):321-326.
- Hallahan DE, Spriggs DR, Beckett MA, et al. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation.Proc Natl Acad Sci U S A. 1989;86(24):1010410107.
- Friedman EJ. Immune modulation by ionizing radiation and its implications for cancer immunotherapy.Curr Pharm Des. 2002;8(19):17651780.
- Sridharan V and Schoenfeld JD. Immune effects of targeted radiation therapy in cancer.Discov Med. 2015;19(104):219-228.
- Ganss R, Ryschich E, Klar E, et al. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication.Cancer Res. 2002;62(5):14621470.
- Cameron RB, Spiess PJ, Rosenberg SA. Synergistic antitumor activity of tumor-infiltrating lymphocytes, interleukin 2, and local tumor irradiation. Studies on the mechanism of action.J Exp Med. 1990;171(1):249263.
- Weichselbaum RR, Hallahan DE, Beckett MA, et al. Gene therapy targeted by radiation preferentially radiosensitizes tumor cells.Cancer Res. 1994;54(16):4266 4269.
- Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases following treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer.Clin Cancer Res. 2005;11(2 Pt 1):728734.
- Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody.Clin Cancer Res. 2009:15(17):5379-5388.
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma.N Engl J Med. 2012;366(10):925-931.
- Chandra RA, Wilhite TJ, Balboni TA, et al. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab.Oncoimmunol. 2015;4(11):e1046028.
- Hallahan DE, Vokes EE, Rubin SJ, et al. Phase I dose-escalation study of tumor necrosis factor-alpha and concomitant radiation therapy.Cancer J Sci Am. 1995;1(3):204209.
