Improved Sensitivity, Monitoring Needed for MRD in Adult and Pediatric ALL
Despite advances with MRD monitoring, further research and practice advances are needed to continue the trend for improving outcomes in patients with acute lymphoblastic leukemia.
Lori Muffly, MD, MS

Minimal residual disease (MRD) monitoring detects and quantifies leukemia cells after treatment intervention and predicts the risk for relapse posttreatment (ie, chemotherapy, immunotherapy, radiotherapy, transplantation).1-3 Negative MRD is the treatment goal in adult acute lymphoblastic leukemia (ALL) and a clinical measure in clinical trials.4 The consensus from North American experts recommends using detection methods with a sensitivity of at least 10-4 to properly assess MRD.5
“[MRD testing] is not new or novel but it has to be part of our arsenal for every patient with ALL,” said Lori Muffly, MD, MS, assistant professor of medicine in the Division of Blood and Marrow Transplantation, Stanford Medicine, in California. Muffly addressed the role of MRD in ALL during the Society of Hematologic Oncology 2021 Annual Meeting.
“Over the last several years, MRD testing in ALL has become the standard not only in just pediatric ALL, but for adults with ALL. [MRD is] universally accepted in ALL; it’s in every guideline, and it’s below the standard of care to not test MRD in ALL,” Muffly said. The National Comprehensive Cancer Network (NCCN) guideline on ALL acknowledges that the incorporation of MRD testing improved the cure and survival rates in patients with ALL.6
But despite advances with MRD monitoring, further research and practice advances are needed to continue the trend for improving outcomes in patients with ALL by exploring issues such as increased testing sensitivity, the need for MRD monitoring after achieving MRD negativity, the appropriate samples for MRD monitoring, and MRD testing for leukemia in the central nervous system.
“There are interesting questions moving forward and worth evaluating that are related to monitoring for patients in remission, using peripheral blood or other sources for MRD monitoring, and determining the optimal sensitivity threshold in patients with ALL,” Muffly said.
Increasing Sensitivity of MRD Testing
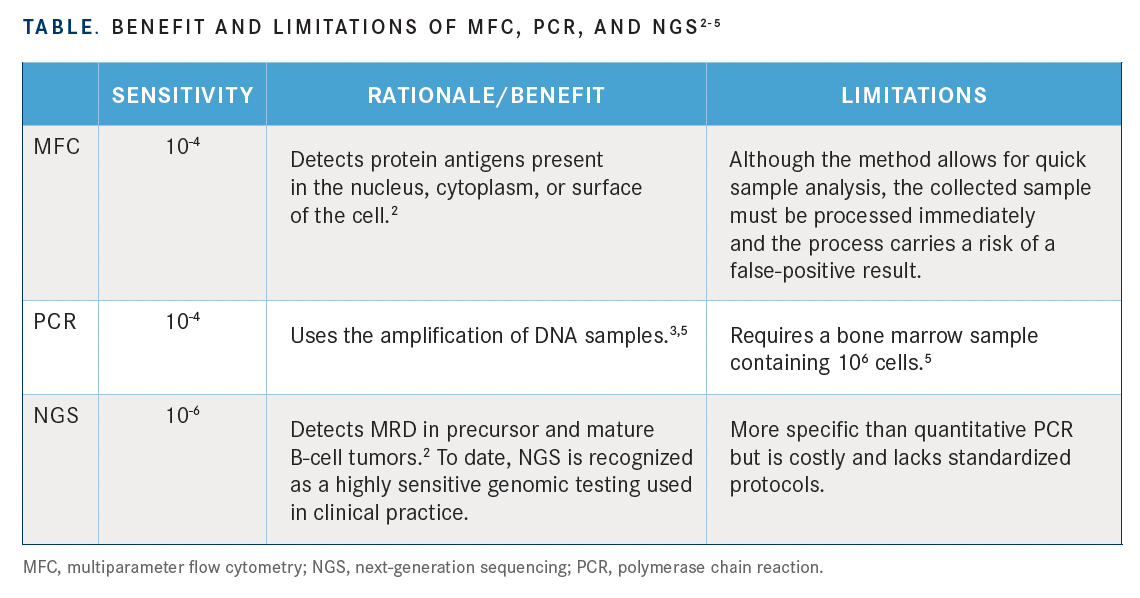
Clinicians can choose from a variety of diagnostic methods to determine MRD: multiparameter flow cytometry (MFC), quantitative polymerase chain reaction (PCR), next-generation sequencing (NGS), and others.3,5,7 The benefits and limitations are highlighted in the TABLE.
The consensus from North American experts recommends using PCR over MFC for patients with Philadelphia chromosome–positive ALL.5 In patients with Philadelphia chromosome–negative ALL, the choice of a method for MRD testing depends on access to an assay, a laboratory, and an experienced pathologist.
“The take-home [message] is that one of these assays really needs to be performed probably with every bone marrow that is assessed after treatment,” said Muffly. Moreover, according to Muffly, “MRD assays on the bone marrow really must have a sensitivity of at least 10-4 and be performed by a validated laboratory.”
Unfortunately, achieving MRD negativity does not translate into the complete absence of residual disease and frequently, MRD is defined as measurable residual disease instead of minimal residual disease.2 When discussing MRD testing methods, Muffly said: “Will sensitivity continue to increase? We all have patients [with] MRD negativity at 10-6—which is essentially as sensitive as our assays get at this time—who relapse. But what are we missing?” Remissions are common in patients with ALL, with some data suggesting that up to 45% of patients with an initial response to ALL relapse eventually.1,8
Available studies highlight that methods with higher sensitivity can detect MRD that remained undetected by methods with lower sensitivity. In a study, NGS identified additional 55 patients with B-cell ALL who were positive for MRD, but MFC failed to detect MRD positivity in these patients.9 The additional patients with MRD positivity represented 9% of the total study population and 38.7% of the patients with MRD positivity at a level of least 10-4. The patients with MRD positivity based on NGS, but not on MFC, experienced a decreased 5-year event-free survival compared with patients whose MRD negativity was confirmed by both MFC and NGS (P = .036). According to another retrospective study, NGS detected MRD positivity in additional 32 samples that tested negative using MFC.10 Strikingly, only 8% of the patients with MRD negativity determined by NGS relapsed after 5 years, compared with 27% of the patients with MRD negativity tested by MFC. The patients with MRD positivity detected by NGS but not by MFC also experienced a decreased 5-year survival rate—67% vs 100% survival rate for patients with MRD negativity confirmed by both MFC and NGS. Muffly wonders: “Will assays continue to have increased sensitivity and is there a threshold at which we can really capture all residual leukemia? I don’t have an answer to that.” But detecting MRD at a higher sensitivity level, using methods such as NGS, calls for a sufficient amount of DNA from sample cells.2 And thus, the assessment and interpretation of MRD results heavily rely on detection methods and their limitations.
MRD Monitoring After Achieving MRD Negativity
Numerous studies have shown that achieving MRD negativity leads to improved outcomes in ALL. In a meta-analysis of 23 studies, adults with B-cell ALL who achieved MRD negativity experienced an improvement in relapse-free survival (random effects hazard ratio [HR]), 2.34; 95% confidence interval [CI], 1.91-2.86) and overall survival (HR, 2.19; 95% CI, 1.63-2.94) compared with patients who were positive for MRD.11 This impact of MRD negativity remained regardless of the disease stage, MRD sensitivity threshold, timing of MRD testing, Philadelphia chromosome status, or MRD detection method (MFC or PCR).
In another meta-analysis of 39 studies with 13,637 pediatric and adult patients with ALL, MRD negativity also led to an improvement in event-free survival compared with MRD positivity status, with 10-year event-free survival rates of 77% vs 32% in pediatric patients and 64% vs 21% in adult patients, respectively.12 Similar to the previous meta-analysis, MRD negativity status carried benefits despite the detection method (MFC or PCR), cutoff values, and timing of MRD monitoring.
“MRD testing after delivering frontline therapy is standard of care and is expected,” Muffly said. According to the consensus recommendation from the North American experts, patients receiving frontline treatment for ALL require MRD assessment from bone marrow at the end of induction, after 3 months of the consolidation therapy, and then every 3 months for at least 3 years in most cases.5 Patients undergoing hematopoietic cell transplantation (HCT) benefit from MRD assessment before HCT and every 3 months after HCT.
MRD Negativity
The question remains whether ongoing MRD monitoring plays a role once patients have achieved treatment response and MRD negativity. A few studies have attempted to address this issue.
A prospective study found that 27% of adults with ALL who have achieved MRD negativity after receiving various chemotherapy combinations eventually converted to MRD positivity during the early post-consolidation phase.13 After about 9.5 months since testing positive for MRD, patients experienced clinical relapse.
Another study revealed that 55 out of 546 patients with ALL who initially achieved MRD negativity with treatment (hyper-CVAD [cyclophosphamide, vincristine sulfate, doxorubicin hydrochloride, and dexamethasone]-based regimens) eventually progressed to MRD positivity.14 Clinical relapse occurred about 3.6 months after MRD relapse. Thus, both studies concluded that the conversion to MRD positivity may predict relapse in patients who have initially achieved disease response and MRD negativity.13,14
“[It is important] to understand the benefit of serial MRD monitoring, in which relapses are no longer a surprise because you are continuously looking for leukemia for a certain amount of time. I think that it’s a really important future step in ALL,” Muffly said. “At our center, that is what we do for patients who undergo cellular therapies. We monitor them every couple of months using NGS or PCR of the peripheral blood, and we found that approach to be very useful.”
Peripheral Blood vs Bone Marrow Samples
Bone marrow or peripheral blood serve as the typical sources for MRD monitoring, with peripheral blood carrying lower levels of MRD compared with bone marrow, especially in patients with B-cell ALL.2,15,16
NGS can use peripheral blood to assess MRD, which facilitates more frequent monitoring, less invasive procedure, and improved patient experience.4 But some concerns exist around samples from peripheral blood contributing to MRD detection at a lower sensitivity level compared with samples from bone marrow.4,17
“In ALL, there has been less data looking at peripheral blood. Our group has recently done a couple of different studies. In a recent study prospectively comparing peripheral blood and bone marrow samples in adults with ALL using next generation sequencing assay, we saw an excellent correlation between peripheral blood and bone marrow,” Muffly said. In the prospective study, MRD status measured by NGS obtained from peripheral blood versus bone marrow were highly correlated (r = 0.87) in 69 patients with ALL undergoing HCT.18 “Our center has substituted peripheral blood for monitoring purposes, particularly posttransplant. I think that there is likely, in the future, to be a clear role for peripheral blood, perhaps not as a sole disease assessment but certainly for ongoing monitoring, particularly for patients in remission. It’s a much more patient-friendly way to monitor, and you can measure peripheral blood MRD as frequently you feel is necessary,” Muffly said.
MRD Testing for ALL in Central Nervous System
“I think the question of how to best monitor for central nervous system leukemia and whether flow cytometry or other MRD assays would add to our ability to detect disease, to monitor the disease, and to prognosticate is really important,” Muffly said. Recent studies explored a relationship between markers of leukemia in central nervous systems and relapse. In one study, the presence of lymphoblasts in cerebrospinal fluid (CSF) detected by MFC revealed an increased risk for relapse in patients with ALL, with a 4-year cumulative incidence of relapse of 16.7% vs 5.1%.19 Another study determined that the presence of lymphoblasts in CSF (also called CSF positivity) in patients with ALL receiving hyper-CVAD, detected by MFC, corresponded to a 3-year cumulative incidence of central nervous system relapse of 15% vs 3.3% of patients who maintained CSF negativity.20 Thus, further research may explore whether MRD assays may play a role when analyzing CSF negativity vs positivity.
References:
1. Contreras Yametti GP, Ostrow TH, Jasinski S, Raetz EA, Carroll WL, Evensen NA. Minimal residual disease in acute lymphoblastic leukemia: current practice and future directions. Cancers (Basel). 2021;13(8):1847. doi:10.3390/cancers13081847
2. Kim IS. Minimal residual disease in acute lymphoblastic leukemia: technical aspects and implications for clinical interpretation. Blood Res. 2020;55(S1):S19-S26. doi:10.5045/br.2020.S004
3. Kruse A, Abdel-Azim N, Kim HN, et al. Minimal residual disease detection in acute lymphoblastic leukemia. Int J Mol Sci. 2020;21(3):1054. doi:10.3390/ijms21031054
4. Muffly L. Assessment of measurable residual disease (MRD) in adult patients with acute lymphocytic leukemia: best use and a case report. Clin Adv Hematol Oncol. 2020;18(suppl 9):10-14.
5. Short NJ, Jabbour E, Albitar M, et al. Recommendations for the assessment and management of measurable residual disease in adults with acute lymphoblastic leukemia: a consensus of North American experts. Am J Hematol. 2019;94(2):257-265. doi:10.1002/ajh.25338
6. NCCN. Clinical Practice Guidelines. Acute lymphoblastic leukemia, version 2.2021. Accessed August 6, 2021. https://www.nccn.org/professionals/physician_gls/pdf/all.pdf
7. Muffly L. Minimal residual disease monitoring of acute lymphoblastic leukemia by high-throughput sequencing of the peripheral blood: case examples and literature review. Clin Lymphoma Myeloma Leuk. 2018;18(suppl 1):S53-S55. doi:10.1016/j.clml.2018.06.055
8. Ganzel C, Wang XV, Rowe JM, et al. At three years, patients with acute lymphoblastic leukaemia are still at risk for relapse. Results of the international MRC UKALLXII/ECOG E2993 trial. Br J Haematol. 2020;191(1):37-43. doi:10.1111/bjh.16616
9. Wood B, Wu D, Crossley B, et al. Measurable residual disease detection by high-throughput sequencing improves risk stratification for pediatric B-ALL. Blood. 2018;131(12):1350-1359. doi:10.1182/blood-2017-09-806521
10. Short N, Kantarjian H, Patel K, et al. Ultrasensitive next-generation sequencing-based measurable residual disease assessment in Philadelphia chromosome-negative acute lymphoblastic leukemia after frontline therapy: correlation with flow cytometry and impact on clinical outcomes. Presented at: 62nd American Society of Hematology Annual Meeting and Exposition; December 7, 2020; virtual. Accessed August 1, 2021. https://ash.confex.com/ash/2020/webprogram/Paper141971.html
11. Bassan R, Brüggemann M, Radcliffe HS, Hartfield E, Kreuzbauer G, Wetten S. A systematic literature review and meta-analysis of minimal residual disease as a prognostic indicator in adult B-cell acute lymphoblastic leukemia. Haematologica. 2019;104(10):2028-2039. doi:10.3324/haematol.2018.201053
12. Berry DA, Zhou S, Higley H, et al. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol. 2017;3(7):e170580. doi:10.1001/jamaoncol.2017.0580
13. Raff T, Gökbuget N, Lüschen S, et al. Molecular relapse in adult standard-risk ALL patients detected by prospective MRD monitoring during and after maintenance treatment: data from the GMALL 06/99 and 07/03 trials. Blood. 2007;109(3):910-915. doi:10.1182/blood-2006-07-037093
14. Pemmaraju N, Kantarjian H, Jorgensen JL, et al. Significance of recurrence of minimal residual disease detected by multi-parameter flow cytometry in patients with acute lymphoblastic leukemia in morphological remission. Am J Hematol. 2017;92(3):279-285. doi:10.1002/ajh.24629
15. Coustan-Smith E, Sancho J, Hancock ML, et al. Use of peripheral blood instead of bone marrow to monitor residual disease in children with acute lymphoblastic leukemia. Blood. 2002;100(7):2399-2402. doi:10.1182/blood-2002-04-1130
16. van der Velden VH, Jacobs DC, Wijkhuijs AJ, et al. Minimal residual disease levels in bone marrow and peripheral blood are comparable in children with T cell acute lymphoblastic leukemia (ALL), but not in precursor-B-ALL. Leukemia. 2002;16(8):1432-1436. doi:10.1038/sj.leu.2402636
17. Sala Torra O, Othus M, Williamson DW, et al. Next-generation sequencing in adult B cell acute lymphoblastic leukemia patients. Biol Blood Marrow Transplant. 2017;23(4):691-696. doi:10.1016/j.bbmt.2016.12.639
18. Muffly L, Sundaram V, Chen C, et al. Monitoring measurable residual disease using peripheral blood in acute lymphoblastic leukemia: results of a prospective, observational study. Presented at: 62nd American Society of Hematology Annual Meeting and Exposition; December 5, 2020; virtual. Accessed August 1, 2021. https://ash.confex.com/ash/2020/webprogram/Paper138988.html
19. Thastrup M, Marquart HV, Levinsen M, et al. Flow cytometric detection of leukemic blasts in cerebrospinal fluid predicts risk of relapse in childhood acute lymphoblastic leukemia: a Nordic Society of Pediatric Hematology and Oncology study. Leukemia. 2020;34(2):336-346. doi:10.1038/s41375-019-0570-1
20. Garcia KLA, Stevenson PA, Cherian S, et al. Multiparameter flow cytometry (MFC) of cerebrospinal fluid (CSF) from adults receiving Hypercvad for acute lymphoblastic leukemia/lymphoma (ALL) identifies patients at higher risk of central nervous system (CNS) relapse: a single-center retrospective analysis. Presented at: 62nd American Society of Hematology Annual Meeting and Exposition; December 7, 2020; virtual. Accessed August 1, 2021. https://ash.confex.com/ash/2020/webprogram/Paper137780.html