Hormone Receptor, pCR May Point to Survival on Dual HER2 Blockade
Updated data reported from a phase 3 trial show that patients with HER2- positive early breast cancer had better rates of overall survival after experiencing a pathologic complete response on HER2-targeted therapy, and these responses were observed most often in those with hormone receptor–negative status.
Paolo G. Nuciforo, MD, PhD

Updated data reported from a phase 3 trial show that patients with HER2- positive early breast cancer had better rates of overall survival (OS) after experiencing a pathologic complete response (pCR) on HER2-targeted therapy, and these responses were observed most often in those with hormone receptor (HR)–negative status. The results from the NeoALTTO trial (NCT00553358) were reported at the 12th European Breast Cancer Conference by Paolo G. Nuciforo, MD, PhD, 1 who is principal investigator of the Molecular Oncology group at Vall d’Hebron Institute of Oncology in Barcelona, Spain, and may support the use of biomarker-driven approaches for patient selection with anti-HER2 agents.
“The neoadjuvant study with dual HER2 blockade in early HER2[-positive] breast cancer has the longest clinical follow-up, at almost 10 years,” versus other trials of its kind in this patient population, said Nuciforo. “Our results support the design of future trials in HER2-positive breast cancer [to] use pCR as an early efficacy readout of long-term benefit to escalate or de-escalate therapy, particularly for hormone receptor– negative tumors.”
The study was initially performed to determine whether dual HER2 blockade with trastuzumab (Herceptin) and lapatinib (Tykerb) in the neoadjuvant setting would induce improved pCRs in patients with HER2-overexpressing primary breast cancer versus single blockade.
The experimental arms consisted of daily lapatinib at 1500 mg (n = 154) or lapatinib plus weekly trastuzumab at 2 mg/kg following a 4-mg/kg loading dose (n = 152). The comparator consisted of trastuzumab at the indicated dose (n = 149). Patients treated on all 3 arms also received weekly paclitaxel at 80 mg/kg for 12 weeks. After surgery, all patients received 3 cycles of 5-fluorouracil, epirubicin, and cyclophosphamide followed by the same anti-HER2 therapy as assigned in the neoadjuvant phase. Patients with HR-positive status also started endocrine therapy at that time, noted Nuciforo.
Previously reported data indicated that pCR rates were significantly higher for the dual blockade group (51.3%) than the trastuzumab-alone group (29.5%; P = .0001). These trends were observed in women with both HR-positive (41.6% vs 22.7%) and HR-negative (61.3% vs 36.5%) disease.2
“When we look at response according to hormone receptor status, we can see that the most significant results were achieved in patients in the hormone receptor–negative subgroup,” said Nuciforo.
Nuciforo reported on secondary end points of OS and event-free survival (EFS) by treatment arm as well as by achievement of pCR at a median follow-up of 9.7 years. The analysis adjusted for patient’s tumor size, nodal status, HR status, and surgical status. “These analyses represent the final update of the 3-year and 6-year survival outcome data that were previously presented in 2014 and 2019,” Nuciforo said.
Patients’ baseline characteristics were well balanced in the 3 arms. About half of patients (51.0%) on the trial were in the HR-negative subgroup. Most patients (84.2%) had N0/1 lymph node status, and more than half (60.2%) had tumors of 5 cm or less. About twothirds of patients (71.4%) were not candidates for conservation surgery.
In the entire patient population, 9-year EFS rates with lapatinib plus trastuzumab, single-agent lapatinib, and single-agent trastuzumab were 69%, 63%, and 65%, respectively. Compared with trastuzumab, neither the dual HER2 blockade (P = .55) nor the single-agent lapatinib (P = .93) arms reached statistically significant differences. These data were seen in both the HR-positive and -negative groups.
The OS rate at 9 years was 80% with lapatinib plus trastuzumab versus 76% with trastuzumab alone (hazard ratio, 0.79; 95% CI, 0.46-1.34; P = .38). Investigators observed neither a numerically nor statistically significant difference between the single-agent lapatinib and trastuzumab arms. As was true of the EFS analysis, HR status did not have a significant effect on these findings.
“When we looked at the association of survival and pathologic complete response independently of the treatment arm, we performed a landmark analysis [that included patients] if they were event free and still on follow-up 30 weeks after randomization,” said Nuciforo. For this analysis, pCR was defined as complete disappearance of the tumor in the breast and axilla. The model was then adjusted for stratification factors.
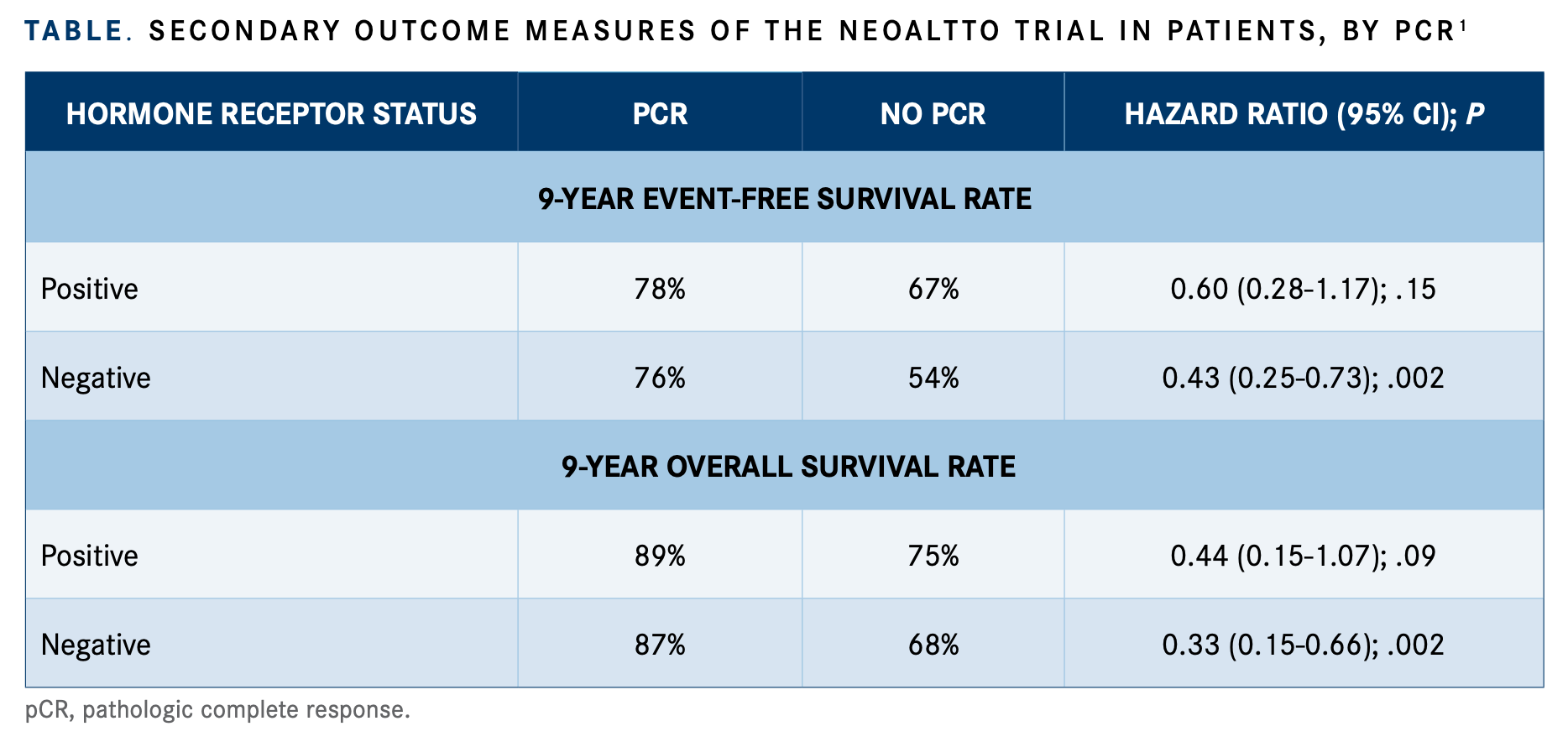
The investigators found that 9-year EFS was significantly associated with those achieving pCR versus those without, with corresponding rates of 77% and 61% (hazard ratio, 0.48; 95% CI, 0.31-0.73; P = .0008). According to results by HR status, the significance of achieving pCR on 9-year EFS rates was upheld in both groups, but rates were more pronounced in the HR-negative subgroup (TABLE).1
Similarly, women achieving pCR had better rates of OS at 9 years versus those without (88% vs 72%, respectively), and this association was statistically significant (hazard ratio, 0.37; 95% CI, 0.20-0.63; P = .0004). Analysis showed a larger OS effect with the achievement of pCR in those with HR-negative disease.
Nuciforo reported that no further concerns over adverse effects have been realized since the 6-year OS data were read out.
“Long-term survival analysis of the NeoALTTO study confirms that patients who achieve pathologic complete response have a significantly higher survival probability than patients with no pCR,” said Nuciforo. “This difference was mainly observed in the hormone receptor–negative tumors and in patients treated with dual [HER2] blockade.”
Reference:
1.Nuciforo P, Townend J, Saura C. Nine-year survival outcome of neoadjuvant lapatinib with trastuzumab for HER2-positive breast cancer (NeoALTTO, BIG 1-06): final analysis of a multicentre, open-label, phase 3 randomised clinical trial. Euro J Can. 2020;138(suppl 1):S15-S16. doi: 10.1016/S0959-8049(20)30560-8
2. Baselga J, Bradbury I, Eidtmann H, et al; NeoALTTO Study Team. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379(9816):633-640. doi:10.1016/S0140-6736(11)61847-3