High Responses, Safety Are Reviewed for Liso-Cel in FL
Treatment with liso-cel had a manageable safety profile with no new safety signals in patients with relapsed/ refractory follicular lymphoma.
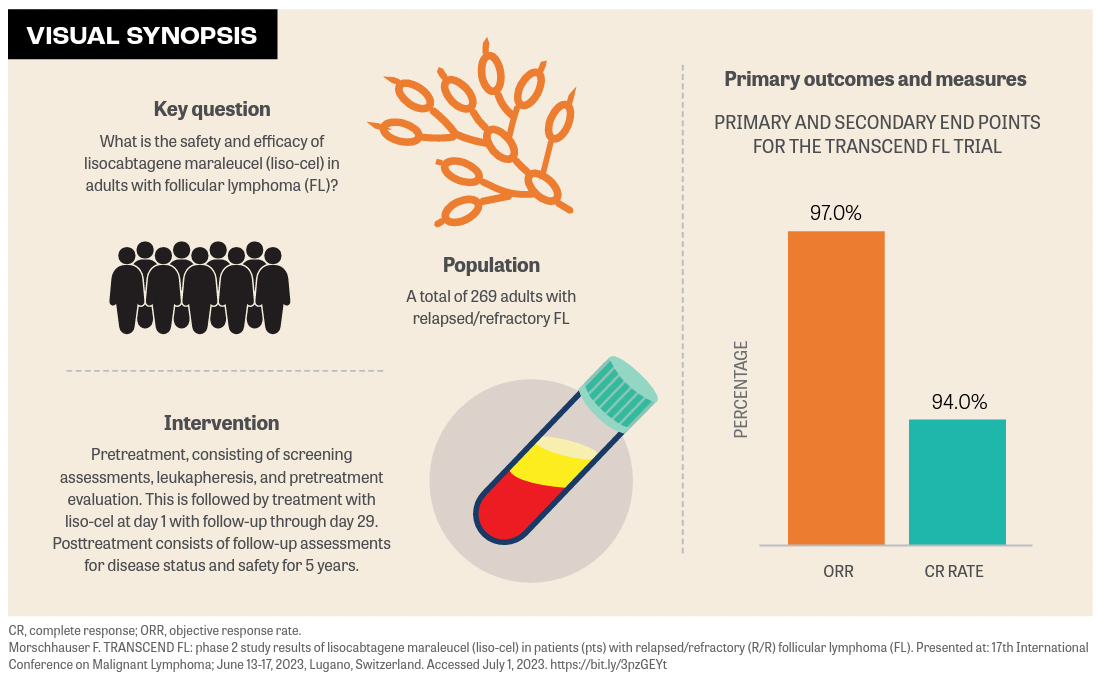
Deep and durable remissions were observed in patients with high-risk follicular lymphoma (FL) who received lisocabtagene maraleucel (liso-cel; Breyanzi), according to findings from the phase 2 TRANSCEND FL trial (NCT04245839) that were presented during the 17th International Conference on Malignant Lymphoma in Lugano, Switzerland. Investigators reported that the study’s primary and secondary end points, objective response rate and complete response rate, were reached at 97% (95% CI, 91.6%-99.4%; P < .0001) and 94% (95% CI, 87.5%-87.8%; P < .0001), respectively.
Franck Morschhauser, MD, PhD, professor of hematology at Hospital Claude Huriez, Lille, France, reported on the primary analysis of patients with relapsed/refractory FL who had received 2 or more prior lines of therapy (third-line efficacy set) and safety (second-line efficacy set).
At 16.6 months the median duration of response (DOR) was not reached and at 12 months, the median DOR was 82%. Similarly, at 17.5 months, median progression-free survival (PFS) was not reached, and at 12 months, median PFS was 80.7%.
“Beyond 50 months, the number of patients at risk was low. We need more mature data to [define] the durability of response,” said Morschhauser.
Patients were eligible to enroll if their FL diagnosis was histologically confirmed with PET-positive and measurable disease 6 months or less before screening, if they had received a combination of anti-CD20 antibody and an alkylator agent, and if they had an ECOG performance status of 0 or 1.
After screening, patients underwent lymphodepletion, which included 30 mg/m2 of fludarabine and 300 mg/m2 of cytarabine. After lymphodepletion, liso-cel was infused for 2 to 7 days at a dose of 100 × 106 chimeric antigen receptor (CAR) T cells. On-study follow-up was 5 years and long-term follow-up was up to 15 years after liso-cel infusion.
The median age of patients in the thirdline cohort (n = 107) was 62 years (range, 23-80 years), the majority were male (62%), and the majority had grade 2 disease (67%). Fifty-seven percent of patients had highrisk disease (grade 3-5), 32% had intermediate risk (grade 2), and 11% had low risk (grade 0-1). Sixty-four percent of patients were double refractory and 41% received bridging therapy.
Regarding safety, the most common grade 3 or higher treatment-emergent adverse events (TEAEs) in the second-line efficacy set (n = 130) were cytopenias with neutropenia at 58%, followed by lymphopenia and leukopenia (both at 13%), and anemia and thrombocytopenia (both at 10%). The most common all-grade TEAEs were neutropenia (65%), cytokine release syndrome (CRS; 58%), anemia (38%), and headache (29%).
AEs of special interest included CRS and neurological events (NEs). Any-grade CRS was 58% and grade 1 was 42%, with a median time to onset of 6 days (range, 1-17) and a median time to resolution of 3 days (range, 1-10). Anygrade NE was 15%, with 12% of patients experiencing grade 1 NEs. Median time to onset was 8.5 days (range, 4-16) and median time to resolution was 3.5 days (range, 1-17).
A total of 28% of patients who experienced CRS were treated with tocilizumab (Actemra) or a combination of tocilizumab and corticosteroids. Patients who experienced CRS and NEs received tocilizumab and corticosteroids (15%), tocilizumab only (11%), or corticosteroids only (2%).
“Prolonged cytopenia defined by grade 3 or more on day 29 was reported in 22% of patients,” Morschhauser said. “But recovery to grade 2 or less was observed in most patients.”
There was 1 death preinfusion and 12 deaths postinfusion, Morschhauser said. Notably, in the postinfusion group, 4 patients progressed, 2 patients developed a new malignancy, and 2 patients died from COVID-19. “Only the macrophage- activating syndrome and the progressive multifocal leukoencephalopathy were considered by the investigators as potentially related to the drug,” Morschhauser said.
“Regarding cellular kinetics, liso-cel exhibited early rapid expansion, with the median time to reach the maximum transgene level around 10 days. Importantly, persistence of liso-cel was observed up to 18 months after infusion in the 22 of 49 evaluable patients with follow-up ongoing,” Morschhauser said.
In the third-line group, the majority (76%) of patients developed B-cell aplasia at baseline. Morschhauser noted that a proportion of patients with B-cell aplasia increased postinfusion, which was maintained above 90% through month 3, then decreased at month 6, but remained constant through month 18.
“Liso-cel had a favorable and manageable safety profile in patients with relapsed/ refractory FL and no new safety signals were observed. These findings support liso-cel as a potential new treatment option for these patients,” Morschhauser concluded.
REFERENCE
Morschhauser F. TRANSCEND FL: phase 2 study results of lisocabtagene maraleucel (liso-cel) in patients (pts) with relapsed/refractory (R/R) follicular lymphoma (FL). Presented at: 17th International Conference on Malignant Lymphoma; June 13-17, 2023, Lugano, Switzerland. Accessed July 1, 2023. https://bit.ly/3pzGEYt

Does Odronextamab Show Hope in FL and DLBCL Despite Regulatory Hurdles?
November 5th 2024Despite regulatory challenges from the FDA, odronextamab has received European approval for the treatment of patients with relapsed/refractory follicular lymphoma or diffuse large B-cell lymphoma following 2 prior treatments.
Read More
Phase 3 Trial of Tafasitamab in Follicular Lymphoma Meets Primary End Point
August 16th 2024The phase 3 inMIND trial evaluating tafasitamab in combination with lenalidomide and rituximab in relapsed or refractory follicular lymphoma showed promising progression-free survival findings, according to topline results.
Read More