Growth Factors Play Emerging Role in Bladder Cancer
When checkpoint inhibitors<strong> </strong>were introduced, it had been more than 30 years since any new drugs had been approved for the treatment of urothelial cancer. Recently, 2 new non–checkpoint inhibitors, erdafitinib and enfortumab vedotin-ejfv, reached the market, and more may be available soon.
Petros Grivas, MD, PhD

Petros Grivas, MD, PhD
When checkpoint inhibitorswere introduced, it had been more than 30 years since any new drugs had been approved for the treatment of urothelial cancer.1Recently, 2 new noncheckpoint inhibitors, erdafitinib (Balversa) and enfortumab vedotin-ejfv (Padcev), reached the market, and more may be available soon.
Despite the benefit immune checkpoint inhibitors (ICIs) have delivered in the advanced setting for frontline cisplatin-ineligible disease, as well as in the platinum-refractory setting, there remains a significant number of patients who do not respond. Looking beyond ICIs, investigators have focused on EGFR, HER2, HER3, and FGFR3 receptors. Erdafitinib (Balversa) and enfortumab vedotin-ejfv (Padcev) are 2 non-ICIs that have gained recent entry into the market, but more are on the way.
Sumanta Kumar Pal, MD

Sumanta Kumar Pal, MD
Altered growth factors and signaling pathways have been found to be drivers of specific types of bladder cancer, saidSumanta Kumar Pal, MD, asso­ciate clinical professor in the Department of Medical Oncology and Therapeutics Research and codirec­tor of the kidney cancer program at City of Hope in Duarte, California, during an interview withTargeted Therapies in Oncology(TTO). Epidermal growth factor receptor (EGFR, also calledERBB1) is involved in sig­naling pathways that control how cells grow and divide.EGFRoverexpression results in ideal conditions for malignant growth, with increased angiogenesis and decreased apoptosis.2 Potentially actionable alter­ations in genes such asERBB2(HER2),ERBB3(HER3), and fibroblast growth factor receptor 3 (FGFR3) are present in up to 60% of high-grade urothelial cancers.3
Patients with muscle-invasive bladder cancer (MIBC) have a relatively high rate ofERBB2alterations, with higher rates found only in breast and gastric cancers, who also frequently haveERBB2overexpression.
“FGFR3is also an important one,” Pal said. “It seems to be a driver in about 20% of patients with bladder cancer; at least, that’s how often we find that the pro­tein gets mutated.”
The challenge: identifying which patients would benefit from treatments that target these growth factor receptors.
“In the past, attempts we have had to target these pathways have been unsuccessful, mainly because we have not been able to select the patients properly,” explainedPetros Grivas, MD, PhD, a medical oncol­ogist at Seattle Cancer Care Alliance in Washington, associate professor of oncology at the University of Washington (UW) School of Medicine in Seattle, and clinical director of the genitourinary cancers program at UW Medicine. I think it’s a patient selection issue, as well as having the appropriate drugs and biomark­ers,” he said during an interview withTTO.
EGFRPositivity in Epithelial Tumors
Overexpression ofEGFRis present in approximately 50% of bladder cancer cases. It is also found in these cancer types: nonsmall cell lung (40%-80%), colorec­tal (25%-77%), advanced gastric (33%), pancreatic (30%-50%), ovarian (35%-70%), breast (15%-30%), and prostate (40%).3
“With lung cancer therapy, investigators are furthest along because they have recognized and targeted multiple altered proteins,” Pal said. “They are well characterized, so you can triage and allocate therapy on the basis of the specific mutations and genes that result in abnormal proteins. It’s different in bladder cancer, whereFGFR3is the only gene that is mutated [and has] an associated FDA-approved prod­uct at this point.”
Grivas agreed with Pal: “I do not think that we had successes in targetingEGFRin urothelial can­cer, maybe because of the lack of proper patient selection. Moreover, the frequency and the driving nature [of] those alterations might be different in urothelial cancer.”
The primary goal of treatment for non-MIBC (NMIBC) is prevention of progression to MIBC. Standard initial treatment for NMIBC is transurethral resection of blad­der tumor (TURBT), immediately followed by adjuvant intravesical chemotherapy. Depending on the patient’s risk level, short-term or ongoing treatment with intra­vesical Bacillus Calmette-Guérin (BCG) may be offered, with radical cystectomy reserved for BCG-ineligible patients or those with the highest risks of recurrence and progression. Systemic chemotherapy with cispla­tin plus gemcitabine or accelerated (dose-dense) MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin) has long been the standard first-line treatment for metastatic urothelial carcinoma and is also given to fit patients prior to radical cystectomy. Attempted blad­der preservation with maximum TURBT followed by chemoradiation is used in selected patients. Systemic therapies for advanced disease include platinum-based chemotherapy; immune checkpoint inhibitors, such as pembrolizumab (Keytruda), atezolizumab (Tecentriq), nivolumab (Opdivo), avelumab (Bavencio), and durvalumab (Imfinzi); and the recently approved erdafitinib and enfortumab vedotin-ejfv.4-6
“Until now, we were limited in treatment options for bladder cancer,” said Arlene O. Siefker-Radtke, MD, a professor in the Department of Genitourinary Medical Oncology, Division of Cancer Medicine, at The University of Texas MD Anderson Cancer Center in Houston. “We had cisplatin-based chemotherapy as the best standard,” she toldTTO.
Newly Approved Options for Oncologists
Recently, oncologists gained new tools for their armamentarium: targeted therapy with erdafitinib or enfortumab vedotin-ejfv. In April 2019, the FDA granted accelerated approval to erdafitinib, a pan- FGFR tyrosine kinase inhibitor, for the treat­ment of locally advanced or metastatic urothelial carcinoma with activatingFGFR3orFGFR2alterations that has progressed during or after platinum-based chemotherapy.7
Enfortumab vedotin-ejfv is an antibody-drug conju­gate that targets NECTIN4, a transmembrane protein expressed in over 80% of bladder cancers. In December 2019, this agent also received accelerated FDA approval for the treatment of patients with locally advanced or metastatic urothelial carci­noma who had already received platinum-based che­motherapy and a PD-1 or PD-L1 immune checkpoint inhibitor.8In EV-103, 45 patients treated with the com­bination of enfortumab vedotin-ejfv and pembrolizum­ab were evaluated for safety and efficacy. After a median follow-up of 11.5 months, the study results continue to meet outcome measures for safety and demonstrate encouraging clinical activity for this plati­num-free combination in the first-line setting. Updated results were presented February 14 during an oral session at the 2020 Genitourinary Cancers Symposium in San Francisco, California.9
One of the biggest problems with chemother­apy regimens, however, is the level of toxicity, particularly in patients with bladder cancer, who tend to be older. “There was great excitement in the field and great hope with the immune check­point inhibitors,” Siefker-Radtke continued. “The original trials suggested a 40% objective response rate [ORR]. But with larger studies, we saw the response rate drift down to around 20%. I think that was a sign of the field being desper­ate for new agents and better options, so even a response rate of 20% was a wonderful thing.”
The erdafitinib approval was based on data from BLC2001, a multicenter, open-label, phase II study of patients with locally advanced, unre­sectable, or metastatic urothelial carcinoma who had progressed on or after at least 1 prior chemotherapy regimen and had at least 1 of a prespecified list ofFGFR3mutations orFGFR2/3gene fusions (FIGURE).10The primary end point was ORR, with progression-free survival (PFS), duration of response (DOR), and overall survival (OS) as secondary end points. The main cohort of 99 patients received continuous erdafitinib dosing beginning at 8 mg once daily. After 2 weeks, the dosage was increased to 9 mg daily in the 41% of patients whose serum phosphate levels (a biomarker of FGFR inhibition) were below 5.5 mg/dL and who had no treatment-related adverse events (TRAEs). Treatment con­tinued until disease progression or unacceptable toxicity. The results showed an ORR of 40%; 3% achieved a complete response (CR), and 37% had a partial response. Median PFS was 5.5 months, and median OS was 13.8 months. Twenty-two of the patients had previously received immunotherapy; their response rate was 59%.10
Toxicity from erdafitinib was an issue in the study, with grade 3 or higher TRAEs reported in 46% of patients. AEs were mainly managed with dose adjustments but caused 13% of patients to discontinue treatment.10
“My perspective on this is that the only other true treatment alternatives we have for bladder cancer management are immune ther­apy or, potentially, chemotherapy,” Pal said.
“Chemotherapy is no walk in the park. It’s chal­lenging for patients, particularly elderly patients. If I were to put targeted therapies on that spec­trum, I would say that targeted therapy is still much easier to tolerate than chemotherapy.”
Erdafitinib caused central serous retinopathy in 21% of the patients in the BLC2001 study; 76% of these cases were resolved with dose interruption or reduction or by administering a concomitant medication. The most common AEs were hyperphosphatemia and stomatitis, affecting 77% and 58% of patients, respectively.10
When the FDA granted accelerated approval for erdafitinib, it also approved Qiagen'stherascreenFGFR RGQ PCR Kit. This laboratory test can identify whether a tumor hasFGFR2orFGFR3mutations or fusions and thus whether the patient is eligible for treatment with erdafitinib.7,11
“This supports the fact that even though we still treat most bladder tumors the same way, there are some tumors with fundamental differ­ences,” Siefker-Radtke said. “As we learn how to target those differences, we can head toward more of a personalized approach and find the best treatment for the right patient.”
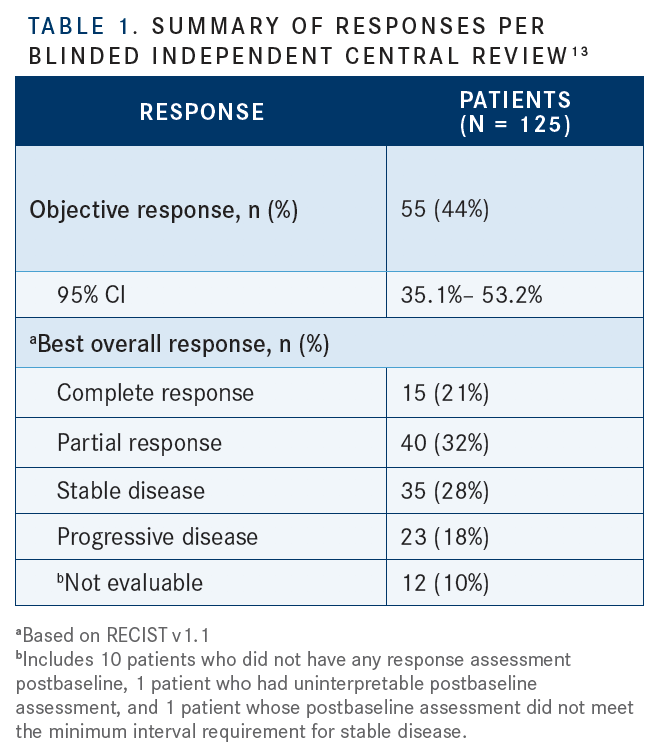
The other drug to recently receive accelerated FDA approval, enfortumab vedotin, does not require testing for mutations or other biomark­ers.12“You don’t have to test for the presence of NECTIN4 [expression] because it’s present at such a high rate across the urothelial cancers,” Siefker- Radtke said. The approval was based on the EV-201 trial, a global, single-arm, phase II study of patients with locally advanced or metastatic urothelial carcinoma who had already undergone treatment with a PD-1 or PD-L1 inhibitor and platinum-based chemotherapy. The primary end point was ORR; secondary end points included DOR, PFS, OS, safety, and tolerability. Follow-up ranged from 0.5 to 16.5 months, with a median of 10.2 months. The confirmed ORR was 44%, including 12% CRs (TABLE 1).13Patients who had liver metastases and those in other prespecified subgroups showed responses similar to those of the overall cohort. Median DOR was 7.6 months, and patients continued treatment until disease progression or unacceptable toxicity.13
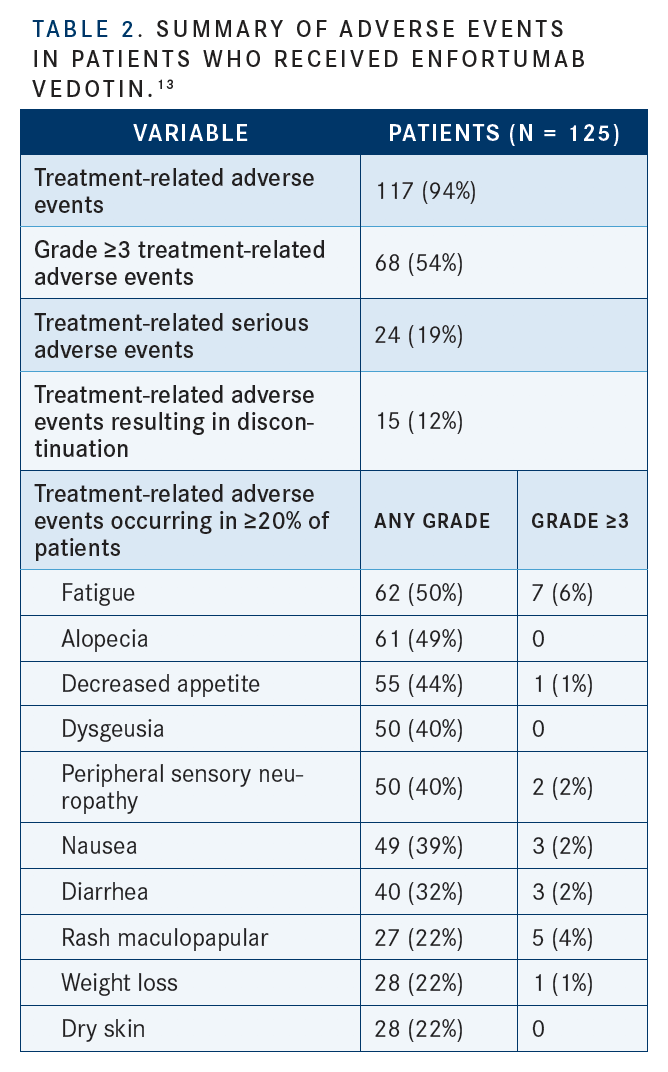
The most common any-grade TRAEs were fatigue (50%), any peripheral neuropathy (50%), alopecia (49%), any rash (48%), decreased appetite (44%), dysgeusia (40%), and peripheral sensory neuropathy (40%) (TABLE 2).13Siefker- Radtke pointed out that, based on the higher diabetic ketoacidosis rates seen in earlier studies, enfortumab vedotin-ejfv may not be a good option for patients with poorly controlled diabetes. These patients also have a higher risk of neuropathy. As a result, the company that manufactures enfortumab vedotin-ejfv has advised that physicians monitor their patients’ hemoglobin A1C levels, she said.
Pushing for Better Targets, More Sequencing
Research continues to produce more and better targeted therapies for urothelial cancer. Grivas mentioned several of the FGFR inhibitors being studied in clinical trials: rogaratinib, which is undergoing a phase II/III trial (NCT03410693); pemigatinib (NCT03914794); infigratinib (NCT04197986); vofatamab (NCT02401542, NCT03123055; both terminated); Debio1347 (NCT01948297, NCT03834220); and AZD4547 (NCT02546661).
“A phase III, multicenter, double-blind, ran­domized, placebo-controlled study evaluating the efficacy of oral infigratinib as adjuvant treatment following surgery in adult subjects with invasive urothelial carcinoma and sus­ceptibleFGFR3genetic alterations is opening in several centers,” he added [NCT04197986]. “I think there is also a single-arm phase II study with pemigatinib in the adjuvant setting in Europe. Other compounds are mainly tested in the metastatic setting, while there is also interest in testing [pemigatinib] in NMIBC.”
Some agents are also being evaluated in noninvasive disease because of the high rates of bladder cancer recurrence and progres­sion to muscle-invasive disease. According a 2013 study by Karim Chamie, MD, MSHS, and colleagues nearly three-fourths of patients with high-grade NMIBC experience recur­rence within 10 years of diagnosis. Although 41% of recurrences occur without progression, 33% progress to muscle-invasive disease.14
Given the new direction in treating urothe­lial cancer, Siefker-Radtke encouraged physicians to pursue mutation testing for their patients with bladder cancer: “With the approval of an FGFR-targeted agent, I would argue that everyone who you think is going to tolerate treatment or tolerate an oral pill like erdafitinib should be tested for the presence of [these] mutations, since the drug can help control their disease.” Educating physicians is key, Pal agreed. “It’s important to promote awareness [about] the importance of gene sequencing and bladder can­cer,” he said. “We put out a physician statement several years ago from the American Society of Clinical Oncology on gene sequencing and bladder cancer. I we were ahead of the curve in the sense that it’s been several years since we began using targeted therapies, but those targeted therapies are having a big impact.”
References
- Kiss B, Wyatt AW, Douglas J, et al. Her2 alterations in muscle-invasive bladder cancer: patient selection beyond protein expression for targeted therapy. Sci Rep. 2017;7:42713. doi: 10.1038/srep42713.
- Colquhoun AJ, Mellon JK. Epidermal growth factor receptor and bladder cancer. Postgrad Med J. 2002;78(924):584-589. doi: 10.1136/pmj.78.924.584.
- Rouanne M, Loriot Y, Lebret T, Soria JC. Novel therapeutic targets in advanced urothelial carcinoma. Crit Rev Oncol Hematol. 2016;98:106-115. doi: 10.1016/j.critrevonc.2015.10.021.
- Bukhari N, Al-Shamsi HO, Azam F. Update on the treatment of metastatic urothelial carcinoma. Scientific World Journal. 2018;2018:5682078. doi: 10.1155/2018/5682078.
- Sylvester RJ, van der Meijden APM, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urology. 2002;168(5):1964-1970. doi: 10.1097/01. ju.0000034450.80198.1c.
- O’Regan T, Tatton M, Lyon M, Masters J. The effectiveness of BCG and interferon against non-muscle invasive bladder cancer: a New Zealand perspective. BJU Int. 201:116, supplement 3, 54-60. doi: 10.1111/bju.13211.
- FDA grants accelerated approval to erdafitinib for metastatic urothelial carcinoma. FDA website. bit.ly/2H7XDY3. Updated April 12, 2019. Accessed February 10, 2020.
- FDA grants accelerated approval to enfortumab vedotin-ejfv for metastatic urothelial cancer. FDA website. bit.ly/2UEbdtZ. Updated December 19, 2019. Accessed February 10, 2020.
- Seattle Genetics and Astellas announce updated results from phase 1b/2 trial of Padcev (enfortumab vedotin-ejfv) in combination with immune therapy pembrolizumab as investigational first-line treatment for advanced bladder cancer [press release]. Bothell, Washington, and Tokyo, Japan: Seattle Genetics, Inc, and Astellas Pharma Inc; February 10, 2020. bit.ly/2HbwhjG. Accessed February 11, 2020.
- Loriot Y, Necchi A, Park SE, et al; BLC2001 Study Group. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Eng J Med. 2019;381(4):338- 348. doi: 10.1056/NEJMoa1817323.
- therascreen FGFR RGQ PCR Kit P180043. May 10, 2019. FDA website. bit.ly/31FMlDT. Updated May 10, 2019. Accessed February 2, 2019.
- Targeting Nectin-4 in bladder cancer. Cancer Discov. 2017;7(8):OF3. doi: 10.1158/2159-8290.CD-NB2017-095.
- Rosenberg JE, O’Donnell PH, Balar AV, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/ programmed death ligand 1 therapy. J Clin Oncol. 2019;37(29):2592-2600. doi: 10.1200/JCO.19.01140.
- Chamie K, Litwin MS, Bassett JC, et al; Urologic Diseases in America Project. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer. 2013;119(17):3219-3227. doi: 10.1002/cncr.28147.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More