GPRC5D-Targeted Therapy Continues to be Effective After Dose Reduction in R/R Multiple Myeloma
At a live virtual event, Leyla Shune, MD, discussed the emergence of talquetamab-tgvs for patients with relapsed/refractory multiple myeloma.
Leyla Shune, MD
Associate Professor, Hematologic Malignancies and Cellular Therapeutics
KU Medical Center
University of Kansas
Kansas City, KS

At a live virtual event, Leyla Shune, MD, associate professor of hematologic malignancies and cellular therapeutics at the University of Kansas Medical Center, discussed the emergence of talquetamab-tgvs (Talvey) for patients with relapsed/refractory multiple myeloma (RRMM). This bispecific monoclonal antibody is a GPRC5D-directed CD3 T-cell engager that has proven its efficacy in this patient population and widened the treatment pool for physicians. Shune discusses the efficacy behind the drug’s approval as well as further research that shows the dose reduction strategy has proved beneficial for maintaining responses and lowering toxicities.
REVIEWING THE MONUMENTAL-1 STUDY
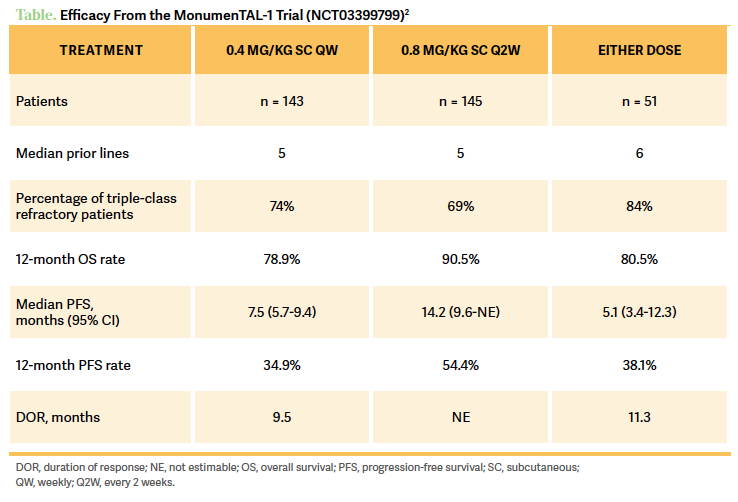
The MonumenTAL-1 trial [NCT03399799] is a phase 1/2 study that looked at talquetamab in patients who had 3 or more prior lines of therapy.1 There were different [study arms], including [patients given talquetamab] subcutaneously weekly, and these people have not received any prior B-cell maturation agent [BCMA] therapy. Then we have the [patients who were on talquetamab] every 2 weeks, [and again they had] no prior BCMA therapy. And then they had a cohort of [patients given talquetamab at 0.4 mg/kg weekly or 0.8 mg/kg] every 2 weeks for patients who have received prior anti-BCMA therapy, chimeric antigen receptor [CAR] T-cell therapy, bispecific T-cell engagers [BiTEs], etc. [In my opinion], what it does is that it negates the disadvantage [from having these other therapies beforehand], because [talquetamab] has a completely different target. But this cohort is a more representative cohort of patients we are taking care of this day and age, as many are heavily pretreated.1
Looking at the efficacy [of talquetamab], patients on the weekly dose, who only received maybe a prior antibody-drug conjugate, had a very good partial response [VGPR] rate of 59.4%.2 Patients on the 0.8-mg/kg dose given every 2 weeks had a VGPR rate of 60.7%, and [talquetamab] was well received [in patients who had a prior] CAR T-cell therapy as well [Table2]. The most common nonhematological adverse events [AEs] of any grade [for patients on the 0.8-mg/kg dose given every 2 weeks] included dry mouth [40.0%], infection [66.2%]—but not that severe or frequent—skin- [73.1%] and nail- [53.8%] related changes, weight loss [41.4%] from pure intake, and rash [29.7%]. [Hematologic-related grade 3/4 AEs for patients on the weekly 0.4-mg/kg dose included] mostly neutropenia [7.7%] and then infections [1.4%].2
USES OF TALQUETAMAB
[In a few cases] we have used talquetamab to bridge [patients] into CAR T-cell therapy. I think initially, people are very worried about the AEs here, but we see that we have a bigger problem with infection with a BCMA-targeted bias. We feel that GPRC5D is a different target than our current [options]. So we use it as a bridging therapy because we’re honestly desperate and we hope that it doesn’t affect the downstream CAR T-cell effect, and we also use it after CAR T-cell therapy [in some cases].
[Ultimately], I think that the target is so different that prior history with T-cell therapy does not negate it, so [the patient isn’t] affected by prior CAR T-cell therapy exposure. It’s almost like being on even ground, and they can [both] still work in this patient. GPRC5D is expressed on the plasma cells but also can be found on [other cells in the] hair shaft, nails, and on the tongue. There’s talk about GPRC5D being [expressed] on the olivary nucleus in the medulla oblongata, but I don’t think that immune effector cellassociated neurotoxicity syndrome can be correlated to that, to be honest.
DOSE REDUCTION PROVES EFFECTIVE
[In this study there were also cohorts where] they tried to reduce the dose intensity.3 So, they had 2 cohorts, 1 was prospective, whereby if patients [were being given 0.8 mg/kg of talquetamab] every 2 weeks then they would go down to 0.4 mg/kg every 2 weeks, as long as they had a partial response or better [to treatment]. Then those who were getting 0.8 mg/kg every 2 weeks could also have a lower dose of 0.8 mg/kg every 4 weeks...if they had a PR [or better].3
Those who had a response to the dose reduction did as well as those who had no dose reduction.... So the key thing is [the patient will] have to be responding [to the talquetamab therapy] if you want to attempt a dose reduction, but if you do so while they’re responding, they don’t have any decrease in their responsiveness to talquetamab.3
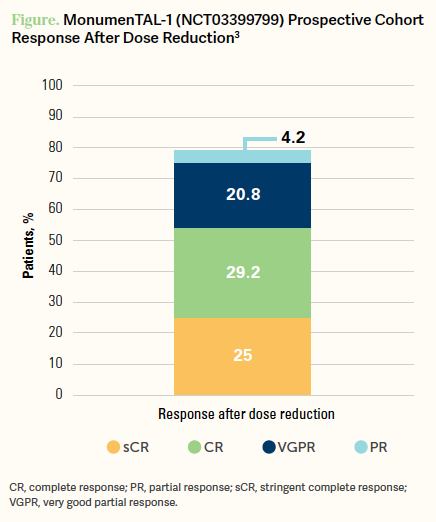
[Looking at the] response rates, the prospective cohort who dose reduced had an overall response rate of 79.2% and a VGPR or better in 75.0% of patients overall [Figure3]. Further, the 12-month progression-free survival [PFS] rate was 50.1% [95% CI, 27.9%-68.7%] and [patients with a dose reduction] had a median PFS of 14.2 months [95% CI, 9.6-not estimable], and compared with the registrational cohort, [these outcomes were] very similar.3 So with dose reductions [there was] a huge improvement with dosing toxicity as nearly every AE got better, except for...weight loss. But overall, patients have had improvement by dropping from 0.8 mg/kg [of talquetamab] given every 2 weeks to 0.4 mg/kg or staying at 0.8 mg/kg but given every 4 weeks.3
TAKEAWAYS WITH TALQUETAMAB
So [dose reduction] is a key way to help your patients tolerate the therapy better [and still] have a good response. On the idea of dose reduction, allowing people to sustain the response just goes to tell you that the dose that we pick on clinical trials is not necessarily what we need because we’re pushing for the maximum tolerated dose. Sometimes that’s not the efficient dose, maybe the efficient dose is way lower, so now, we’re learning how to kind of deescalate [the dose] and still maintain the response.
[When it comes to] AE management we’re getting better, and we’re learning how to decrease the frequency [of the dose] without adding any more barriers.... Once you have the setup in place you can just... piggyback on it for every future BiTE into the same model that you have at your center. The other [thing to highlight] here is the noninferiority, [as this cohort] did not have enough power to show superiority.
They will need way more numbers, like in a post hoc analysis, in the future. With 100-plus patients you can have a better n and a better power, but [as of now] these results are very impressive looking. I feel that drug companies, and all stakeholders, must make it possible for community oncologists to deliver this drug [in the later line settings], because it’s going to be needed everywhere. But this setup is coming because we have a responsibility to teach, help, and deliver [the proper care] for their patients, too.
REFERENCES
1. Chari A, Minnema MC, Berdeja JG, et al. Talquetamab, a T-cell–redirecting GPRC5D bispecific antibody for multiple myeloma. N Engl J Med. 2022;387(24):2232-2244. doi:10.1056/NEJMoa220459
2. Touzeau C, Schinke C, Minnema M, et al. S191: Pivotal phase 2 MonumenTAL-1 results of talquetamab (tal), a GPRC5DXCD3 bispecific antibody (BsAB), for relapsed/refractory multiple myeloma (RRMM). HemaSphere. 2023;7 (suppl):e5955094. doi:10.1097/01.HS9.0000967676.59550.94
3. Chari A, Oriol A, Chamorro MCM, et al. Efficacy and safety of less frequent/lower intensity dosing of talquetamab in patients with relapsed/refractory multiple myeloma: Results from the phase 1/2 MonumenTAL-1 study. Blood. 2023;142(suppl 1):1010. doi:10.1182/blood-2023-181228

Gasparetto Explains Rationale for Quadruplet Front Line in Transplant-Ineligible Myeloma
February 22nd 2025In a Community Case Forum in partnership with the North Carolina Oncology Association, Cristina Gasparetto, MD, discussed the CEPHEUS, IMROZ, and BENEFIT trials of treatment for transplant-ineligible newly diagnosed multiple myeloma.
Read More