FGFR2 Emerges as a Promising Target in Cholangiocarcinoma
Given the frequency of <em>FGFR2</em> alterations in intrahepatic cholangiocarcinoma, investigators have identified it as a potential prognostic indicator of survival and a rational target of systemic cancer therapies.
Arndt Vogel, MD, PhD
Given the frequency ofFGFR2alterations in intrahepatic cholangiocarcinoma, investigators have identified it as a potential prognostic indicator of survival and a rational target of systemic cancer therapies.
Beyond the established frontline therapy of gemcitabine and cisplatin, patients with metastatic cholangiocarcinoma have limited options.1 “For patients with advanced disease, we only have 1 established chemotherapy [that] allows a median overall survival [OS] of around 1 year,” said Arndt Vogel, MD, PhD, professor of gastrointestinal oncology at Hannover Medical School in Germany during a presentation at the European Society for Medical Oncology (ESMO) 2019 Congress. “Second-line therapy is not well established.”
FGFR fusions are present in up to 15% of cases of intrahepatic cholangiocarcinoma and represent an emerging biomarker for therapy. Several presentations at the conference focused on outcomes related to aberrations in this gene and therapies that may be effective as targeted agents.
FGFR2Gene Fusions in Cholangiocarcinoma
Findings from the prospective, observational PRELUDE study by Masafumi Ikeda, MD, and colleagues presented during the meeting showed that certain patient characteristicssuch as age <65 years (P= .032), alcohol consumption >60 g/day (P= .023), and hepatitis B or C positive status (P= .012)were associated withFGFR2fusionpositive intrahepatic and perihilar cholangiocarcinoma. The results also showed thatFGFR2fusions were present in 7.7% of patients with intrahepatic cholangiocarcinoma.2
A presentation by Daniel R. Almquist, MD, and colleagues showed that somatic FGFR2 fusions may be a prognostic indicator for survival and chemotherapy response. In a cohort of 135 patients with intrahepatic cholangiocarcinoma, the investigators identified 45 that harbored FGFR2 fusions. Similar to the PRELUDE findings, the results showed an association with younger age, though it was not statistically significant. However, there was a greater likelihood for patients with these aberrations to have advanced disease stage IIIb or greater.3
Efficacy with gemcitabine-platinum chemotherapy was also evaluated in both patient subgroups showing no significant difference in progression-free survival (PFS; HR, 1.19;P= .36). However, patients harboring FGFR2 fusions had significant improvements in median OS at 2.7 years versus 1.3 years in patients with no such aberration (HR, 0.44;P= .002).3
“FGFR is a therapeutic target of interest, where future prospective studies will be necessary to validate the predictive, prognostic utility and its relevance in patient outcomes in intrahepatic cholangiocarcinoma,” Almquist et al, wrote in their study.
Results of the FIGHT-202 Trial
The open-label, single-arm phase II FIGHT-202 trial was undertaken in the United States, Europe, Middle East, and Asia to evaluate the efficacy of pemigatinib, a selective oral inhibitor of FGFR1/2/3, in patients with locally advanced or metastatic cholangiocarcinoma despite at least 1 line of prior therapy. All patients had theirFGF/FGFRstatus centrally confirmed and were required to have adequate renal function. Patients in 3 cohorts were treated with oral pemigatinib at 13.5 mg daily using a 2-weeks-on/ 1-week-off schedule (FIGURE).4,5
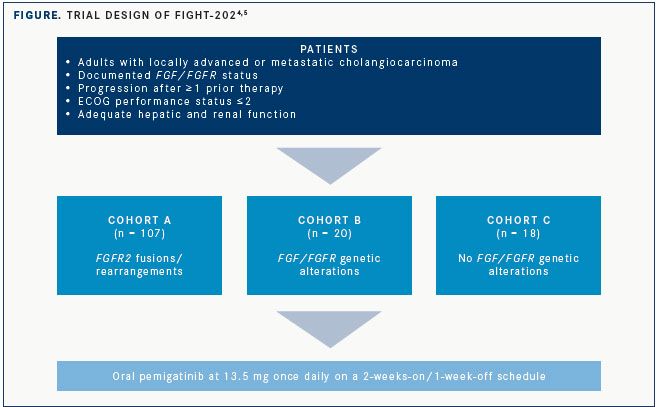
Results were presented at the ESMO 2019 Congress and showed that treatment with pemigatinib in patients with previously treated, locally advanced or metastatic cholangiocarcinoma with anFGFR2rearrangement or fusion induced an objective response rate (ORR) of 35.5%.
More than one-third of 107 total patients withFGFR2fusions/rearrangements (cohort A) responded to therapy, and the median duration of response (DOR) was 7.5 months (95% CI, 5.7-14.5). In contrast, there were no responses observed in the 20 patients enrolled with otherFGF/FGFRgenetic alterations (cohort B) or the 18 with noFGF/FGFRalterations (cohort C).
The higher ORR translated to a longer median PFS in cohort A. Median PFS was 6.9 months (95% CI, 6.2-9.6) in cohort A compared with 2.1 months (95% CI, 1.2-4.9) in B and 1.7 months (95% CI, 1.3-1.8) in C.
OS data in cohort A were not yet mature at the time of data cutoff (March 22, 2019), but with a median duration of follow-up of 15.4 months and a median duration of treatment of 7.2 months, the median OS was 21.1 months (95% CI, 14.8-not evaluable) in the cohort withFGFR2fusions/rearrangements. Median OS was 6.7 months (95% CI, 2.1-10.6) in cohort B after a median follow-up of 19.9 months and median duration of treatment of 1.4 months. In cohort C, the median OS was just 4.0 months (95% CI, 2.3-6.5) after a median follow-up of 24.2 months and median duration of treatment of 1.3 months.
“Overall, I think these data indicate that FGFR inhibition is a meaningful treatment for this genetically defined subgroup of patients with cholangiocarcinoma,” said Vogel, who is the lead investigator on the trial.
The study was not designed to make statistical comparisons between the 3 study cohorts. The primary end point was the confirmed ORR in cohort A by independent central review.
A total of 1206 patients screened forFGF/FGFRstatus and 85 with results available were considered for the trial. In total, 127 patients withFGF/FGFRgenetic alterations were included in cohorts A and B. The median age for the entire 146-patient cohort was 59 years (range, 26-78), but those in cohort A tended to be younger, Vogel said. Of those in cohort A, 70% were <65 years compared with 50% in B and 39% in C. Women represented 58% of patients, with 61% in cohort A, 55% in B, and 44% in C. Patients from North America accounted for 61% of the total cohort, 24% were from Western Europe, and 15% were from elsewhere in the world.
Among the 107 patients in cohort A, 92 fusions and 15 rearrangements were discovered. A total of 56 unique fusion partners were identified; the most common was BICC1, which occurred in 29% of patiemts. Forty-two partners were unique to a single patient, and no fusion partner was identified in >5%. “We need a fusion partner agnostic test to find those patients,” Vogel said.
In cohort A, the 35.5% ORR consisted of 3 complete responses (2.8%), 35 partial responses (32.7%), and 50 patients with stable disease (46.7%), for a disease control rate (DCR) of 82%. The ORR was consistent across subgroups.
Adverse events (AEs) were manageable and consistent with the mechanism of action of pemigatinib. The most common AE was hyperphosphatemia, which occurred in 60% of patients, but no grade ≥3 cases were encountered. Hyperphosphatemia was managed with a low-phosphate diet, phosphate binders, diuretics, or dose reduction or interruptions. Just 3 patients required dose reductions/interruptions due to hyperphosphatemia.
Hypophosphatemia of any grade occurred in 23% of patients and was the most common grade ≥3 AE, with a rate of 12%. No case of hypophosphatemia was clinically significant, and none led to treatment discontinuation or dose reduction.
Serous retinal detachment occurred in 4% of patients. “[It] usually resolved spontaneously or after dose interruption,” Vogel said.
Nine percent of patients discontinued due to AEs; the most frequent causes were intestinal obstruction and acute kidney injury (2 each).
Treatment was discontinued in all patients in cohorts B and C and in 76 of 107 patients in cohort A. Progressive disease led to discontinuation in 57 patients in cohort A, 15 in B, and 12 in C; discontinuations due to AEs occurred in 4 patients in cohort A and 2 each in cohorts B and C.
Dose reduction due to AEs was required in 14%, with the most common reasons being stomatitis (n = 5), palmar-plantar erythrodysesthesia syndrome (n = 5), arthralgia (n = 5), asthenia (n = 2), and onychomadesis (n = 2). Some 42% of patients required dose interruptions due to AEs.
Ongoing Phase III Studies of FGFR Inhibitors
Based on the data from FIGHT-202, the phase III FIGHT-302 study of pemigatinib compared with gemcitabine plus cisplatin in the first-line setting in patients with cholangiocarcinoma and FGFR2 fusions/rearrangements is ongoing (NCT03656536), Vogel said. The primary end point is PFS, with secondary end points including ORR, OS, DOR, DCR, and safety.
Another ongoing phase III trial, PROOF, is examining the adenosine triphosphatecompetitive, FGFR1/2/3 selection oral tyrosine kinase inhibitor infigratinib versus gemcitabine plus cisplatin in patients with FGFR2 gene fusions or translocations and cholangiocarcinoma in the frontline setting (NCT03773302). The primary end point is PFS, with secondary end points including ORR, OS, DOR, DCR, and safety.5
Audrey Sternberg contributed to this article.
References:
- Sohal DPS, Shrotriya S, Abazeed M, Cruise M, Khorana A. Molecular characteristics of biliary tract cancer.Crit Rev Oncol Hematol.2016;107:111- 118. doi: 10.1016/j.critrevonc.2016.08.013.
- Ikeda M, Maruki Y, Ueno M, et al. Frequency and clinicopathological characteristics of biliary tract carcinomas harboring the FGFR2fusion gene: a prospective observational study (PRELUDE study).Ann Oncol.2019;30(suppl 5;abstr 723P). doi: 10.1093/annonc/mdz247.050.
- Almquist DR, Javle M, Ciombor KK, et al. FGFR2 fusions and its effect of patient (pt) outcomes in intrahepatic cholangiocarcinoma (iCCA).Ann Oncol.2019;30(suppl 5;abstr 726P). doi: 10.1093/annonc/mdz247.053.
- Vogel A, Sahai V, Hollebecque A, et al. FIGHT-202: a phase 2 study of pemigatinib in patients (pts) with previously treated locally advanced or metastatic cholangiocarcinoma (CCA).Ann Oncol.2019;30(suppl 5;abstr LBA40). doi: 10.1093/annonc/mdz394.031.
- Abou-Alfa GK, Borbath I, Clarke SJ, et al. Infigratinib versus gemcitabine plus cisplatin multicenter, open-label, randomized, phase 3 study in patients with advanced cholangiocarcinoma with FGFR2 gene fusions/translocations: the PROOF trial.Ann Oncol.2019;30(suppl 5;abstr 832TiP). doi: 10.1093/annonc/mdz247.158.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More