Evolving Paradigms in Immuno-Oncology: Checkpoint Inhibitors Under Development For Other Cancers and Summary
This feature covers the "Checkpoint Inhibitors Under Development For Other Cancers" and "Summary" section of the current Evolving Paradigms in Immuno-Oncology issue.
Checkpoint Inhibitors Under Development For Other Cancers
Additional trials to expand currently approved labels, as well as assess the use of investigational agents that target the PD-1/PD-L1 pathways are currently in under way (TABLE 8).
Renal Cell Carcinoma
Inhibition of VEGF via treatment with bevacizumab (Avastin, Genentech) is a common treatment for RCC but may not limit disease progression. As such, alternative treatment strategies are being pursued.104As of November 23, 2015, nivolumab has been approved for the treatment of patients with advanced RCC who have received prior antiangiogenic therapy.24This is based on data from the phase III CheckMate 025 trial in which 821 patients with advanced clear-cell RCC, previously treated with one or two regimens of antiangiogenic therapy, were randomized (1:1) to receive 3 mg/kg nivolumab every 2 weeks or 10 mg everolimus orally once daily. The median age of patients was 62 years (18-88), 75% were male, and 88% were white. The primary endpoint was OS, with secondary endpoints of ORR and safety.105
The median OS was 25.0 months (95% CI, 21.8 to not estimable) for nivolumab compared with 19.6 months (95% CI, 17.6-23.1) with everolimus (HR for death with nivolumab vs everolimus, 0.73; 95% CI, 0.57-0.93; P = .002) indicating superiority of nivolumab treatment. ORR was 25% with nivolumab versus 5% with everolimus (OR, 5.98 [95% CI, 3.68-9.72]; P <.001), and the median PFS was 4.6 months (95% CI, 3.7-5.4) versus 4.4 months (95% CI, 3.7-5.5), respectively (HR, 0.88; 95% CI, 0.75-1.03; P = 0.11). Treatment-related AEs occurred in 79% (n = 319/409) of nivolumab-treated patients and 37% (n = 145/397) of everolimus-treated patients, with grade 3 or 4 AEs occurring in 76% versus 37%, respectively. Fatigue was the most common AE with nivolumab, present in 2% of patients, and anemia (8%) was the most common AE with everolimus. The most common grade 3 or 4 AEs experienced within the nivolumab treatment group were fatigue, anemia, pneumonitis, hyperglycemia, and diarrhea.105
Nivolumab was also assessed in a phase II trial (NCT01354431) of 168 patients with mRCC, randomly assigned (1:1) to receive nivolumab 3 mg/kg (n = 60), 2 mg/kg (n = 54), or 10 mg/kg (n = 54) IV once every 3 weeks. The respective mean PFS per treatment was 2.7, 4.0, and 4.2 months; ORR was seen in 20%, 22%, and 20% of treatment groups, with a mean OS of 18.2 months (80% CI, 16.224.0), 25.5 months (80% CI, 19.8-28.8), and 24.7 months (80% CI, 15.3-26.0). Treatment-related AEs were observed in 75%, 67%, and 78% of patients in the 3 mg/kg, 2 mg/kg, and 10 mg/kg treatment groups, respectively, with grade 3 or 4 AEs seen in 5%, 17%, and 13% of patients in each group, respectively. The most common AEs were fatigue, nausea, pruritus, and rash. A total of 19 patients (11%) experienced grade 3 or 4 AEs, the most common of which were nausea, pruritus, and fatigue.104
Squamous-Cell Carcinoma of the Head and Neck
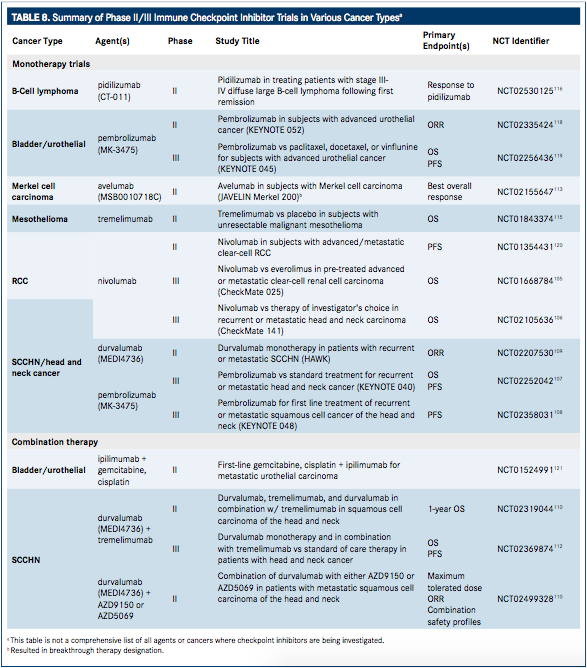
Several checkpoint inhibitor therapies are currently being evaluated in the treatment of squamous-cell carcinoma of the head and neck (SCCHN). Nivolumab is currently in the phase III CheckMate 141 trial (NCT02105636) to determine efficacy compared with investigator choice standard of care in patients with SCCHN following platinum therapy. Patients will be randomized to receive either nivolumab 3 mg/kg every 2 weeks or investigator choice of cetuximab, methotrexate, or docetaxel. The primary outcome measure of this study is OS. The study was initiated May 2014, is set for completion September 2017, and is estimated to enroll 360 patients.106
Pembrolizumab is also currently being investigated in two phase III trials (Keynote 040 and 048) in SCCHN. The Keynote 040 trial (NCT02252042) is a study of 466 patients with platinumrefractory, recurrent SCCHN randomized 1:1 to receive pembrolizumab 200 mg every 3 weeks or investigators' choice standard of care (single-agent methotrexate, docetaxel, or cetuximab). Stratification will be performed based on ECOG PS (0 or 1), human papillomavirus (HPV) status in oropharyngeal cancer by p16 immunohistochemistry testing (positive vs negative), and centralized PDL1 status (positive vs negative). The primary endpoints are PFS and OS. Secondary endpoints are ORR, duration of response, and assessment of PFS, OS, and ORR in PD-L1−positive patients.107In the phase III Keynote 048 study (NCT02358031), pembrolizumab 200 mg every 3 weeks is being evaluated as combination therapy with a platinum-based drug (cisplatin or carboplatin) plus 5-fluorouracil (5-FU) versus cetuximab plus a platinum-based drug (cisplatin or carboplatin) plus 5-FU. This trial was initiated in March 2015, with expected completion in September 2018 over which time an estimated 780 patients will be treated. The primary endpoint is PFS.108
Durvalumab is also being evaluated in phase II trials for the treatment of SCCHN alone or in combination with tremelimumab. In an ongoing, single-arm, phase II monotherapy trial (NCT02207530; HAWK), ORR is being evaluated in patients with recurrent or metastatic PD-L1−positive SCCHN (estimated enrollment of 112 patients). The trial was initiated October 2014 with expected completion in August 2017.109In a randomized, open-label phase II trial (NCT02319044), both durvalumab and tremelimumab are being evaluated as monotherapy, as well as in combination. This trial was initiated in April 2015, and is expected to be completed in December 2017. Estimated enrollment is 240 patients (randomized 1:1:2 to receive durvalumab:tremelimumab:combination), and the primary outcome measure is ORR.110
In a third, randomized, two-part, dose-escalation, open-label phase II trial (NCT02499328), durvalumab is being evaluated in combination with either AZD9150 or AZD5069 (a STAT3 antisense oligonucleotide and chemokine receptor 2 antagonist, respectively) as second-line treatment of SCCHN. The study was initiated in August 2015 with expected completion in May 2017. Nearly 150 patients are expected to be randomized for treatment with AZD9150 plus durvalumab, AZD5069 plus durvalumab, AZD9150 alone, or durvalumab alone. The first part of the study will evaluate patients with advanced solid malignancies refractory to standard therapy for which no standard of care regimen currently exists. The second part will further explore dose expansion for each agent and combination.111
The combination of durvalumab with tremelimumab compared with durvalumab monotherapy for the treatment of SCCHN is also being investigated in a randomized, open-label, multicenter global, phase III study (EAGLE; NCT02369874). An estimated 720 patients with recurrent or metastatic PD-L1−positive or −negative SCCHN will be randomized based on pretreatment assessment of PD-L1 expression per a prespecified cut-off level (25%, with 0%-24% considered negative and >25% positive). Tumor assessments will be performed every 8 weeks for the first 48 weeks and then every 12 weeks thereafter. The primary outcome measures are OS and PFS.112The study was initiated in September 2015 with expected completion in September 2018.
Merkel Cell Carcinoma
In November 2015, the antiPD-L1 antibody, avelumab, was granted breakthrough therapy designation by the FDA for the treatment of Merkel cell carcinoma. This is based on the phase II, open-label, multicenter JAVELIN Merkel 200 study (NCT02155647) initiated in June 2014. The study is set for a primary completion date with data release in January 2016, at which time an estimated 88 patients will have been treated with avelumab 10 mg/kg once every 2 weeks. The primary outcome measure for the study is best overall response. Secondary objectives include assessment of the duration of response, PFS, OS, and safety. Evaluation of the pharmacokinetic profile of avelumab will also be performed along with an exploratory assessment of immune-related responses and evaluation of response rate in relation to PD-L1 expression.113
Mesothelioma
Tremelimumab, an investigational CTLA-4 inhibitor, was recently reported by its manufacturer (AstraZeneca) to have received orphan drug designation for the treatment of malignant mesothelioma,114 and is currently being studied in a phase IIb, randomized, double-blind, parallel-group study (NCT01843374). The study was initiated May 2013 with an estimated completion date in April 2016 over which time approximately 565 patients with mesothelioma will be treated with tremelimumab compared with placebo. The primary outcome measure for this study is OS.115
Other Investigational Checkpoint Inhibitors
Other investigational checkpoint inhibitors currently in development include the antiPD-1 therapy, pidilizumab (CT-011; CureTech), which is currently in phase II (NCT02530125) for B-cell lymphoma,116 and the anti–PD-1 therapy, MEDI0680 (AMP-514), which is currently in phase I development (NCT02118337) in combination with durvalumab in advanced malignancies.117
Summary
Currently, there is a rapid evolutionary advance occurring in cancer care with the approval of several antiPD-1/anti-PD-L1 immunotherapies and the introduction of numerous investigational agents. The strategic immune checkpoint blockade of the PD-1 receptor and its ligand, PD-L1, is in a state of swift development for the current and prospective treatment of several cancers including melanoma, lung, bladder, mesothelioma, renal, and head and neck carcinoma. New biological agents such as durvalumab, avelumab, and atezolizumab are demonstrating progress with positive treatment results and manageable safety profiles. Already approved therapies such as nivolumab and pembrolizumab are also experiencing expansions in indications to include various types of cancers beyond their initial melanoma approval.
Gasparetto Explains Rationale for Quadruplet Front Line in Transplant-Ineligible Myeloma
February 22nd 2025In a Community Case Forum in partnership with the North Carolina Oncology Association, Cristina Gasparetto, MD, discussed the CEPHEUS, IMROZ, and BENEFIT trials of treatment for transplant-ineligible newly diagnosed multiple myeloma.
Read More
Key Trials From ASH 2024 Impact Treatment for Plasma Cell Disorders Going Forward
February 20th 2025Peers & Perspectives in Oncology editorial board member Marc J. Braunstein, MD, PhD, FACP, discussed the significant advancements in multiple myeloma treatment at the 2024 ASH Annual Meeting and Exposition.
Read More