Emerging Targeted Therapies and Immunotherapies in CLL
An overview of emerging targeted therapies and immunotherapies in chronic lymphocytic leukemia.
1The potential synergy of ibrutinib with other treatment strategies, including immunotherapeutic and targeted approaches, is currently being investigated in various clinical trials with the hope of identifying novel combinations that increase responses and improve duration of response.
New targeted agents in development in CLL include second-generation inhibitors of Bruton’s tyrosine kinase (BTK) designed to improve safety and efficacy, and novel inhibitors of phosphoinositide 3-kinase (PI3K).2,3Emerging therapies in CLL also include novel immunotherapeutic regimens using immune checkpoint inhibitors and adoptive immunotherapy using modified T lymphocytes.4
IMPROVING MODULATION OF APOPTOSIS WITH BTK INHIBITION
Venetoclax (Venclexta), a selective inhibitor of the B-cell lymphoma-2 (BCL-2) family of proteins, which regulate cellular apoptosis and oncogenic functions, is a standard-of-care treatment of relapsed or refractory 17p deletion (del[17p]) CLL, alone or in combination with rituximab (Rituxan), and has activity in patients relapsing or refractory to ibrutinib or idelalisib (Zydelig).5The combination of venetoclax and rituximab plus bendamustine in patients with relapsed/refractory CLL, based on findings from the phase III MURANO study.6Pretreating CLL cells with a BTK inhibitor, such as ibrutinib, has been shown to increase the dependence of CLL cells on BCL-2, enhancing CLL killing in response to venetoclax in cell culture studies.7
Supported by this rationale, the clinical utility and safety of a sequenced regimen of ibrutinib monotherapy for 3 cycles followed by combination therapy with ibrutinib and venetoclax is being explored in an ongoing phase II study in 78 patients with relapsed/ refractory CLL and in previously untreated patients with high-risk CLL, defined as the presence of either del(17p), mutated TP53, 11q deletion (del[11q]), unmutated IGHV, or ≥65 years.8A phase II study is also evaluating ibrutinib plus venetoclax as a frontline therapy in a planned cohort of 150 patients with CLL.9
Potential synergy of BTK and BCL-2 inhibition is also being explored in a phase Ib/II study in patients with relapsed/refractory CLL, using a combination of obinutuzumab (Gazyva), ibrutinib, and venetoclax started sequentially, with gradual escalation of the venetoclax dose.10The regimen was tolerated with toxicities consistent with those of the single agents. Response has been evaluated in 6 of 12 patients treated in the phase Ib portion of the study, with an ORR of 100% (5 partial responses [PRs] and 1 CR).10
EXPLORING SYNERGY OF IBRUTINIB WITH IMMUNOTHERAPEUTIC REGIMENS
The upregulation of inhibitory immune checkpoint pathways that control the T-cell response represents a major mechanism by which cancer cells escape elimination by the immune system. CLL cells express elevated levels of checkpoint inhibitory molecules, including programmed death 1 (PD-1), and its main ligands, PD-L1 and PD-L2.11This provides a strong rationale to investigate immunotherapy with PD-1 checkpoint inhibitors in CLL, which is expected to enhance the activity of anti-CLL T- effector cells, resulting in immune-mediated elimination of CLL cells. The small molecule inhibitor ibrutinib has immunomodulatory properties because it also blocks interleukin-2inducible T-cell kinase (ITK), in addition to BTK, producing a shift in the balance of T-helper cell populations.
Adding ibrutinib with PD-1 checkpoint blockade has enhanced antitumor activity in preclinical models, including ibrutinib-resistant lymphomas.12An ongoing phase II trial is investigating combined checkpoint inhibitor therapy with the PD-1 inhibitor nivolumab (Opdivo) and ibrutinib in patients with relapsed/refractory CLL or Richter’s transformation (RT), or untreated patients with high-risk del(17p) CLL.13An initial report of the first 12 patients was presented at the 2016 ASH Annual Meeting, reporting activity in ibrutinib-naive patients with relapsed/refractory CLL (PRs in 3 of 5 patients) or RT (2 of 4 patients), including 1 patient with del(17p), unmutated IGHV, complex karyotype CLL.14
A single-arm phase II study has previously demonstrated activity of ibrutinib in combination with rituximab in patients with high-risk CLL.15The therapy was well tolerated and produced a response rate (ORR) of 95%, including a CR of 8% and a PR of 87%, and an 18-month progression-free survival (PFS) rate of 78% in all patients and 72.4% in patients with del(17p) or a TP53 mutation.15
Promising clinical activity of ibrutinib in combination with the CD20-targeted monoclonal antibody ofatumumab (Arzerra) has been reported from a phase I/II trial in 71 patients with relapsed/ refractory CLL; the combination produced a high ORR (71% to 100% in different dose schedules) and an estimated 12-month PFS of 75% to 89%.16Frontline treatment with ibrutinib plus obinutuzumab is currently being compared with chlorambucil plus obinutuzmab in the phase III iLLUMINATE study in 212 treatment-naive patients with CLL, with results expected in late 2017.17
Ibrutinib has also been combined with the investigational glycoengineered CD20 antibody ublituximab (TG-1101). In a phase II study, 88% of patients (n = 41) with relapsed/refractory CLL responded to the combination, and the ORR was 95% among patients with high-risk (del(17p), del(11q), or TP53 mutation) CLL, including a subset (n = 3, 15%) who achieved negative minimal residual disease (MRD).18
The phase III GENUINE study (n = 126 patients) was designed to validate this combination in patients with previously treated high-risk CLL.19According to recently released top-line data, the study met the primary endpoint by demonstrating a significant increase in ORR with ibrutinib plus ublituximab versus single-agent ibrutinib (80% vs 47%; P <.001).20
“We believe that the rapid responses seen in our phase II study with ublituximab plus ibrutinib are validated here in our phase III GENUINE study and are important markers of improved overall efficacy and patient outcomes,” stated study chair Jeffrey Sharman, MD, medical director for hematology research for the US Oncology Network in a prepared statement.
The combination of ibrutinib with obinutuzumab is the focus of a phase I/II study in 32 previously untreated patients with CLL, with safety, tolerability, dose-limiting toxicity, and ORR as primary outcomes.21
COMBINING AND COMPARING TARGETED THERAPY WITH CHEMOIMMUNOTHERAPY
Recent outcomes from the randomized, placebo-controlled, phase III HELIOS study in patients with relapsed/refractory CLL have confirmed that the addition of ibrutinib to bendamustine and rituximab (BR) was beneficial and tolerable.22After a median follow-up of 17 months, median PFS was not reached in the ibrutinib group versus 13.3 months in the placebo group (HR, 0.203; P <.0001), demonstrating a reduction of the risk of progression and death by 80%. PFS at 18 months was 79% versus 24% (P <.0001), and PFS benefits of ibrutinib were maintained in subgroups including refractoriness to purine analog therapy, del(11q), and unmutated IGHV. The ORR was 82.7% versus 67.8% (P <.0001), and the median OS was not reached in either group.23
Current trials evaluating ibrutinib in combination with chemotherapy as frontline therapy include a phase II study evaluating the addition of short-course udarabine to ibrutinib23and a phase II study investigating ibrutinib plus udarabine, cyclophosphamide, and rituximab (FCR) in younger patients (<65 years).24
An ongoing phase III study of the German CLL Study Group has been designed to directly compare frontline standard chemoimmunotherapy (FCR, BR) therapy in physically fit patients with CLL who do not have del(17p) or TP53 mutations with combination regimens of targeted drugs, specifically rituximab plus venetoclax, obinutuzumab plus venetoclax, and obinutuzumab plus ibrutinib plus venetoclax.24
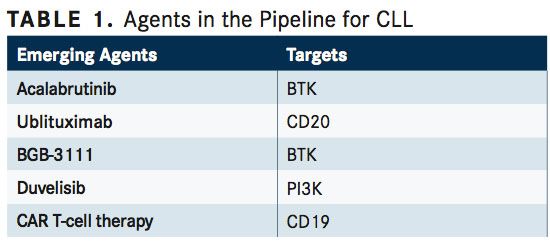
SECOND-GENERATION BTK INHIBITORS
The second-generation small molecule BTK inhibitors acalabrutinib (ACP-196) and BGB-3111 (TABLE 1) exhibit greater selectivity for BTK than ibrutinib, which may reduce toxicity related to off-target activity of ibrutinib on other kinases including EGFR, ITK, and Tec family kinases, and may also overcome drug resistance related to incomplete BTK inhibition with ibrutinib.2Both agents also exhibit rapid oral bioavailability and a short half-life, allowing for twice-daily dosing, which has been associated with high plasma exposureproducing continuous and greater-than-95% blockade of BTK.26,27
An uncontrolled, multicenter, phase I/II trial involving 61 patients with relapsed/refractory CLL, with unmutated IGHV in 75% and del(17p) in 31%, reported a 95% ORR (PR, 85%; PR with lymphocytosis, 10%; stable disease, 5%) with a median follow-up period of 14.3 months. The ORR in patients with del(17p) was 100%, and no cases of Richter transformation had occurred. Most toxicities were grade 1/2, including headache, diarrhea, increased weight, pyrexia, and upper respiratory infection.26
A phase III trial of acalabrutinib versus ibrutinib in previously treated patients with high-risk CLL with either del(17) and/or del(11q) is underway.28A second ongoing phase III trial is evaluating acalabrutinib versus rituximab plus idelalisib or bendamustine in patients with relapsed/refractory CLL.13
Acalabrutinib is also being investigated for the frontline treatment of CLL, based on positive outcomes of a phase I/II study in treatment-naïve patients with CLL, which reported good tolerability and an ORR of 96%.29The ongoing phase III Elevate CLL TN study is comparing acalabrutinib plus obinutuzumab versus obinutuzumab plus chlorambucil as a frontline treatment.30
Phase I study outcomes with BGB-3111 in patients with relapsed/refractory CLL were recently reported and included an ORR of 96% (67% PR; 28% PR with lymphocytosis) and a 100% ORR in patients with del(17p).27Three ongoing phase I trials are evaluating BGB-3111 in patients with B-cell lymphoid malignancies as a single agent and in combination with obinutuzumab and BGB-A317, an early-stage PD-1 inhibitor.32,33
“BGB-3111 can achieve complete target inhibition in lymph nodes while remaining highly tolerable,” said lead investigator Constantine Tam, MD, of the Peter MacCallum Cancer Center, Australia, in a prepared statement.31
NEW PI3K INHIBITORS
Duvelisib (IPI-145) is an oral inhibitor of PI3K3γ and PI3Kδ isoforms that suppresses B-cell proliferation and promotes apoptosis in CLL cells.33Clinical activity of single-agent duvelisib was reported from a phase I trial in patients with relapsed/refractory CLL, including those with del(17p) or a TP53 mutation.35Phase III data of the DUO trial of patients with relapsed/refractory CLL/SLL showed a median PFS of 13.3 and 9.9 months for duvelisib and ofatumumab, respectively (HR, 0.52;P<.0011). Additionally, initial data from an ongoing phase Ib study of duvelisib plus FCR as frontline therapy suggested tolerability and high response rate (100% in 12 patients).37
Outcomes from the phase III DUO study comparing single-agent duvelisib versus ofatumumab in patients with relapsed/refractory CLL are expected later this year.36
CELL-BASED IMMUNOTHERAPY
A unique immunotherapy currently in development in CLL is adoptive immunotherapy using chimeric antigen receptor (CAR) T-cell therapy. This technology involves the genetic modification of autologous T cells ex vivo to express a fusion construct (CAR) consisting of a B cell-specific antigen, such as the pan B-cell antigen CD19, and the signaling component of T-cell receptor.4This approach results in target specificity of the effector T cell, and later-generation designs incorporate additional co-stimulatory domains to enhance antitumor activity of the modified T cells.
Clinical outcomes of CAR T-cell therapy in patients with CLL have been reported in small numbers at single institutions.4The largest triala phase I study—demonstrated a 57% ORR (8 of 14 patients; including 4 CRs and 4 PRs) among patients with heavily pretreated relapsed/refractory CLL.38CD19 CAR T-cell responses persisted for 4 years in the rst 2 patients who achieved CR, and none of the patients with CR relapsed or had detectable MRD, suggesting this treatment may be a possible cure for CLL. All patients with response developed B-cell aplasia and had cytokine release syndrome, which coincided with T-cell proliferation.38
Single-arm results of a small phase I/II study of JCAR014 CAR T-cell therapy in adults with relapsed/refractory B-cell malignancies, which were presented at the 2017 SOHO Annual Meeting, showed that the subgroup of patients who met International Working Group on CLL criteria for response had no malignant IgH sequences in marrow had a PFS and OS of 100% after follow-up of 6.6 months.39
A large number of early-stage clinical trials is ongoing to investigate CAR T-cell treatment in advanced B-cell malignancies, including relapsed/refractory CLL, including a phase I/II study in 189 patients with advanced CLL, acute lymphoblastic leukemia, and non-Hodgkin lymphoma.38
Multiple clinical trials are underway that address ef cacy and tolerability of novel agents and agent combinations in CLL. Pertinent outcomes include the durability of remissions in patients who achieve MRD-negative CR on regimens and the effects of treatment discontinuation.
“The field is rapidly moving into the domain of combination therapies with encouraging early results and apparently higher quality responses including high rates of MRD-negativity,” said Tam and John Seymour, MD, also of Peter MacCallum Cancer Centre and University of Melbourne, in a commentary. “Such combination therapies, including rationally designed novel combinations are now being evaluated in a suite of high-profile trials that will likely define optimal treatment in the near future. If these combination therapies do emerge as superior, we will again need to evaluate outcomes and treatment choices in that context.”
References:
- Woyach JA. How I manage ibrutinib-refractory chronic lymphocytic leukemia. Blood. 2017;129:1270-1274.
- Wu J, Liu C, Tsui ST, Liu D. Second-generation inhibitors of Bruton tyrosine kinase. J Hematol Oncol. 2016;9(1):80. doi: 10.1186/s13045-016-0313-y.
- Olin JL, Canupp K, Smith MB. New pharmacotherapies in chronic lymphocytic leukemia. P T. 2017;42(2):106-115.
- Fraietta JA, Schwab RD, Maus MV. Improving therapy of chronic lymphocytic leukemia with chimeric antigen receptor T cells. Semin Oncol. 2016;43(2):291- 299. doi: 10.1053/j.seminoncol.2016.02.006.
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guide- lines in oncology (NCCN Guidelines) chronic lymphocytic leukemia/small lym- phocytic lymphoma. NCCNorg 2017; Version 2.2017.
- Wierda W, Chyla B, Eichhorst B, et al. Venetoclax in relapsed/refractory chronic lymphocytic leukemia (CLL) with 17p deletion: outcomes and minimal residual disease (MRD) from the full population of the pivotal M13-982 trial. Presented at 2017 SOHO Annual Meeting; September 13-16, 2017; Houston, TX Abstract Cll-102.
- Deng J, Isik E, Fernandes SM, et al. Bruton’s tyrosine kinase inhibition increases BCL-2 dependence and enhances sensitivity to venetoclax in chronic lympho- cytic leukemia [printed ahead of publication February 14, 2017]. Leukemia. doi: 10.1038/leu.2017.32.
- National Institutes of Health Clinical Trials Registry. Venetoclax and ibrutinib in patients with chronic lymphocytic leukemia (CLL). ClinicalTrials.gov website. Identi er: NCT02756897. Updated April 26, 2107. Accessed May 18, 2017.
- National Institutes of Health Clinical Trials Registry. Ibrutinib plus venetoclax in patients with treatment-naive chronic lymphocytic leukemia /small lymphocytic lymphoma. ClinicalTrials.gov website. Identi er: NCT02910583. Updated April 11, 2107. Accessed May 18, 2017.
- Jones JA, Woyach J, Awan FT, et al. Phase 1b Results of a Phase 1b/2 Study of Obinutuzmab, Ibrutinib, and Venetoclax in Relapsed/Refractory Chronic Lympho- cytic Leukemia (CLL). Presented at: 58th ASH Annual Meeting and Exposition; December 2-6, 2016; San Diego, CA. Abstract 639.
- Ramsay AG, Clear AJ, Fatah R, et al. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood. 2012;120:1412-1421.
- Sagiv-Bar I, Kohrt HE, Czerwinski DK, et al. Therapeutic antitumor immunity by checkpoint blockade is enhanced by ibrutinib, an inhibitor of both BTK and ITK. Proc Natl Acad Sci USA. 2015;112:E966-72.
- National Institutes of Health Clinical Trials Registry. Nivolumab with ibrutinib for relapsed, refractory or high-risk untreated patients with chronic lymphocytic leukemia (CLL). ClinicalTrials.gov website. Identi er: NCT02420912. Updated March 31, 2107. Accessed May 18, 2017.
- Jain N, Basu S, Thompson PA, et al. Nivolumab combined with ibrutinib for CLL and Richter transformation: a phase II trial. Presented at: 58th ASH Annual Meet- ing and Exposition; December 2-6, 2016; San Diego, CA. Abstract 59.
- Burger JA, Keating MJ, Wierda WG, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: a single- arm, phase 2 study. Lancet Oncol. 2014;15(10):1090-1099.
- Jaglowski SM, Jones JA, Nagar V, et al. Safety and activity of BTK inhibitor ibruti- nib combined with ofatumumab in chronic lymphocytic leukemia: a phase 1b/2 study. Blood. 2015;126(7):842-850.
- National Institutes of Health Clinical Trials Registry. A multi-center study of ibru- tinib in combination with obinutuzumab versus chlorambucil in combination with obinutuzumab in patients with treatment naïve CLL or SLL. ClinicalTrials.gov web- site. Identi er: NCT02264574. Updated January 4, 2017. Accessed May 18, 2017.
- Sharman JP, Farber CM, Mahadevan D, et al. Ublituximab (TG-1101), a novel glycoengineered anti-CD20 antibody, in combination with ibrutinib is safe and highly active in patients with relapsed and/or refractory chronic lymphocytic leukaemia: results of a phase 2 trial. Br J Haematol. 2017;176(3):412-420.
- National Institutes of Health Clinical Trials Registry. Ublituximab in combination with ibrutinib versus ibrutinib alone in patients with previously treated high- risk chronic lymphocytic leukemia (CLL). ClinicalTrials.gov website. Identi er: NCT02301156. Updated December 13, 2017. Accessed May 18, 2017.
- TG Therapeutics Press Release March 6, 2017. TG Therapeutics announces positive topline data from phase 3 GENUINE study of TG-1101 in combination with ibrutinib in patients with high risk chronic lymphocytic leukemia (CLL). Available at: http:// ir.tgtherapeutics.com/releasedetail.cfm?ReleaseID=1015939. Accessed at May 18, 2017.
- National Institutes of Health Clinical Trials Registry. Ibrutinib in combination with GA101 (Obinutuzumab) in previously untreated chronic lymphocytic leuke- mia (CLL) patients. ClinicalTrials.gov website. Identi er: NCT02315768. Updated March 23, 2017. Accessed May 18, 2017.
- Chanan-Khan A, Cramer P, Demirkan F, et al. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. J Clin Oncol. 2015;33 (suppl; abstr LBA7005).
- National Institutes of Health Clinical Trials Registry. A phase II study using ibrutinib and short-course udarabine in previously untreated patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). ClinicalTrials.gov website. Identi er: NCT02514083. Updated May 17, 2017. Accessed May 18, 2017.
- National Institutes of Health Clinical Trials Registry. A phase II study of ibrutinib plus FCR in previously untreated, younger patients with chronic lymphocytic leukemia (iFCR). ClinicalTrials.gov website. Identi er: NCT02251548. Updated April 10, 2017. Accessed May 18, 2017.
- National Institutes of Health Clinical Trials Registry. Standard chemoimmuno- therapy (FCR/BR) versus rituximab + venetoclax (RVe) versus obinutuzumab (GA101) + venetoclax (GVe) versus obinutuzumab + ibrutinib + venetoclax (GIVe) in fit patients with previously untreated chronic lymphocytic leukemia (CLL) without del(17p) or TP53 mutation (GAIA). ClinicalTrials.gov website. Identi er: NCT02950051. Updated February 8, 2017. Accessed May 18, 2017.
- Byrd JC, Harrington B, O’Brien S, et al. Acalabrutinib (ACP-196) in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):323-332.
- Tam CS, Grigg AP, Opat S, et al. Twice daily dosing with the highly speci c BTK in- hibitor, Bgb-3111, achieves complete and continuous BTK occupancy in lymph nodes, and is associated with durable responses in patients (pts) with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). Presented at: 58th ASH Annual Meeting and Exposition; December 2-6, 2016; San Diego, CA. Abstract 642.
- National Institutes of Health Clinical Trials Registry. Elevate CLL R/R: study of aca- labrutinib (ACP-196) versus ibrutinib in previously treated subjects with high risk chronic lymphocytic leukemia. ClinicalTrials.gov website. Identi er: NCT02477696. Updated November 28, 2016. Accessed May 18, 2017.
- Byrd JC, Jones JA, Furman RR. Acalabrutinib, a second-generation bruton tyrosine kinase (Btk) inhibitor, in previously untreated chronic lymphocytic leukemia (CLL). J Clin Oncol. 2016;34(suppl; abstr 7521).
- Elevate CLL TN: study of obinutuzumab + chlorambucil, acalabrutinib (ACP-196) + obinu- tuzumab, and acalabrutinib in subjects with previously untreated CLL. ClinicalTrials.gov website. Identi er: NCT02475681. Updated February 14, 2017. Accessed May 18, 2017.
- BeiGene. BeiGene presents updated clinical data on BTK inhibitor BGB-3111 in patients with chronic lymphocytic leukemia and small lymphocytic leukemia at the 2016 Amer- ican Society of Hematology annual meeting 2016. Available at: http://ir.beigene.com/ phoenix.zhtml?c=254246&p=irol-newsArticle&ID=2227736. Accessed May 18, 2017.
- National Institutes of Health Clinical Trials Registry. BGB 3111 in combination with obinutuzumab in subjects with B-cell lymphoid malignancies. ClinicalTrials.gov website. Identi er: NCT02569476. Updated August 30, 2016. Accessed May 18, 2017.
- National Institutes of Health Clinical Trials Registry. BGB-3111 in combination with BGB-A317 in subjects with B-cell malignancies. ClinicalTrials.gov website. Identi er: NCT02795182. Updated August 30, 2016. Accessed May 18, 2017.
- Balakrishnan K, Peluso M, Fu M, et al. The phosphoinositide-3-kinase (PI3K)-delta and gamma inhibitor, IPI-145 (duvelisib), overcomes signals from the PI3K/AKT/S6 pathway and promotes apoptosis in CLL. Leukemia. 2015; 29(9):1811-1822.
- O’Brien S, Patel M, Kahl BS, et al. Duvelisib (IPI-145), a PI3K-δ,γ inhibitor, is clinically active in patients with relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;124(21):3334.
- Zinzani P, Wagner-Johnson N, Miller C, et al. DYNAMO: a phase 2 study demonstrating the clinical activity of duvelisib in patients with double-refractory indolent non-Hodgkin lymphoma. Hematol Oncol. 2017; 35(52):69-70./strong>
- Davids MS, Kim HT, Gilbert E, et al. Preliminary results of a phase Ib study of duvelis- ib in combination with FCR (dFCR) in previously untreated, younger patients with CLL. Blood. 2015;126(23):4158.
- National Institutes of Health Clinical Trials Registry. A phase 3 study of duvelisib versus ofatumumab in patients with relapsed or refractory CLL/SLL (DUO). ClinicalTrials.gov website. Identi er: NCT02004522. Updated March 23, 2017. Accessed May 18, 2017.
- Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139.
- National Institutes of Health Clinical Trials Registry. Laboratory treated T cells in treating patients with relapsed or refractory chronic lymphocytic leukemia, non- Hodgkin lymphoma, or acute lymphoblastic leukemia. ClinicalTrials.gov website. Identifier: NCT01865617. Updated April 24, 2017. Accessed May 18, 2017.
- Turtle C, Hay KA, Hanafi LA, et al. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chemieric antigen receptor-modified T cells after failure of ibrutinib. J Clin Oncol. 2017;35(26):3010-3020. doi: 10.1200/JCO.2017.72.8519.