Development of Molecular Models Represents Significant Progress in Management of Medulloblastoma
The management of medulloblastoma, the most common malignant brain cancer in children, has not changed in decades but breakthroughs are on the horizon.
Robert Wechsler-Reya, PhD

Robert Wechsler-Reya, PhD
The management of medulloblastoma (MB), the most common malignant brain cancer in children, has not changed in decades but breakthroughs are on the horizon.
Only 5 years after the definition of the molecular subgroups of MB, several new targets and candidate drugs have been identified. Just as importantly, clinical networks have been established to recruit sufficient numbers of patients with the appropriate MB subtype and treatment histories for trials. Models of MB are also valuable tools for rigorous preclinical testing to ensure that only drugs that are effective and nontoxic advance to testing in patients.
Drugs that target the specific molecular changes driving growth and survival of MB should eradicate the cancer while sparing the rest of the brain and other tissues. As progress has been made toward the discovery of these central mutations, genomic analysis of patient tumors has revealed at least 4 molecular variants, which also have distinct presentations, demographics, and clinical outcomes. Two of these subgroups, Wingless (WNT) and Sonic hedgehog (SHH), have genetic abnormalities in specific pathways, whereas the biology of MB for Group 3 and group 4 is less well understood.
To discover targeted therapies for the more common and severe types of MB (SHH and Groups 3 and 4), our laboratory has led efforts to develop genetically engineered mouse models and patient-derived xenografts for studying the molecular basis of the disease.
SHH MB: Cells of Origin and New Targets
The models we created to study SHH MB allowed us to address an important question in tumor biology: from what cells are MB tumors derived? Identifying the cell of origin enables direct comparisons between tumor cells and their normal counterparts so that tumor vulnerabilities can be identified.
Because inactivation of the Patched (PTCH) gene was known to lead to MB tumors in mice, we deleted PTCH in granule neuron precursors (GNPs) or neural stem cells,1and found that either cell type could give rise to SHH MB. However, when PTCH was deleted in neural stem cells, these cells only generated tumors after they differentiated into GNPs, suggesting that the granule lineage is particularly susceptible to transformation by SHH pathway mutations.
We have also identified several new promising therapeutic targets for SHH MB using the PTCH-mutant model. For example, we identified a population of tumor-propagating cells whose proliferation is driven by aberrant activation of genes that facilitate the switch from the G2 phase of the cell cycle to mitosis.2This result pointed to regulators of G2/M, such as Aurora and polo-like kinases, as targets, and we found that inhibitors of these enzymes block tumor growth in vivo. In another study, we examined the importance of Survivin, a protein that regulates the cell cycle as well as apoptosis, and found that antagonizing this protein suppresses tumor growth.3
New Insight and Translatable Therapies
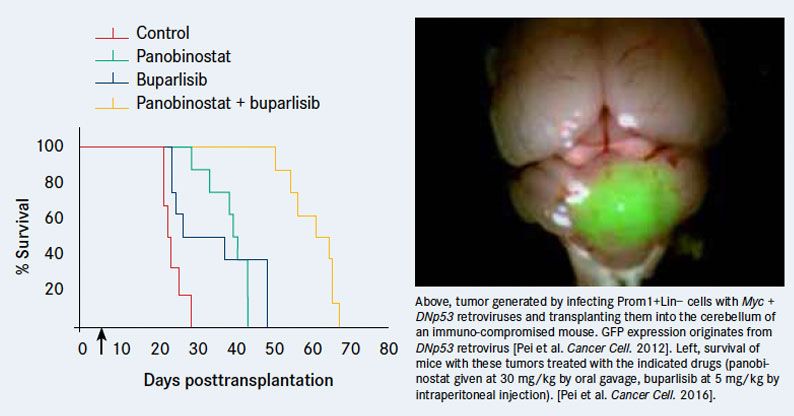
Our research has also produced models of Group 3 MB that have shed light on the molecular basis of this form of MB. In our initial efforts to generate a Group 3 model, we learned that overexpression of MYC, which characterizes these tumors, is insufficient to induce tumor formation on its own.4Generating tumors required combining Myc with another oncogene, such as dominant-negative TP53 (p53 loss is frequently observed in recurrent Group 3 MB), to overcome MYC-induced apoptosis. When neural stem cells expressing both of these genes are transplanted into the cerebellum of naïve mice, they give rise to tumors that resemble human Group 3 MB at a histological and molecular level.Another model resulted from our collaboration with the group of Stefan Pfister, MD, at the German Cancer Research Center in Heidelberg.5Analyzing genome sequences from a large number of patient tumor samples, Pfister and colleagues discovered that in ~30% of Group 3 tumors, growth factor independence 1 (GFI1) or its paralog GFI1B are activated by rearrangements that juxtapose them with active enhancers. We went on to show that neural stem cells expressing MYC and GFI1 (or GFI1B) could generate aggressive tumors that resemble Group 3 MB.
Our models of Group 3 MB have allowed us to identify anticancer drugs that could move rapidly into trials. A high-throughput screen of existing anticancer compounds using primary tumor cells from these mice led to identification of a highly synergistic drug combination: the histone deacetylase inhibitor panobinostat and the PI3K blocker buparlisib markedly impaired tumor growth and prolonged survival of tumorbearing mice.6
Both of these agents are already FDA-approved or in clinical trials for other cancers, so we are currently working with our oncologist collaborators to plan a trial for patients with MYCdriven MB. Given the potential for fast translation, we are now applying this high-throughput screening approach to cells from a large panel of patient-derived xenografts to find new therapies for other MB subgroups.
References:
- Yang ZJ, Ellis T, Markant SL, et al.. Medulloblastoma can be initiated by deletion of Patched in lineage-restricted progenitors or stem cells.Cancer Cell2008;14(2):135-145.
- Markant SL, Esparza LA, Sun J, et al. Targeting sonic hedgehog-associated medulloblastoma through inhibition of Aurora and Polo-like kinases.Cancer Res. 2013;73(20):6310-6322.
- Brun SN, Markant SL, Esparza LA, et al. Surviving as a therapeutic target in Sonic hedgehog-driven medulloblastoma.Oncogene2015;34(29):3770-3779
- Pei Y, Moore CE, Wang J, et al. An animal model of MYC-driven medulloblastoma.Cancer Cell2012;21(2):155-167.
- Northcott PA, Lee C, Zichner T, et al. Enhancer hijacking activates GFI1 family oncogenes in medulloblastoma.Nature. 2014;511(7510):428-434.
- Pei Y, Liu KW, Wang J, et al. HDAC and PI3K antagonists cooperate to inhibit growth of MYC-driven medulloblastoma.Cancer Cell. 2016;29(3):311-323.