Combining Camrelizumab and Chemotherapy Improves Overall Survival in Squamous NSCLC
Results from the CameL-sq clinical trial support camrelizumab plus chemotherapy as a standard first-line treatment option for advanced squamous non–small cell lung cancer, according to Caicun Zhou, MD, PhD.
Caicun Zhou, MD, PhD

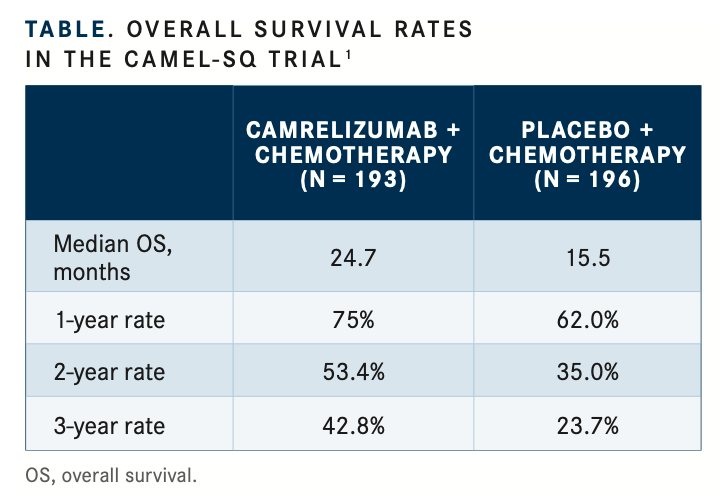
When camrelizumab (formerly SHR-1210) was combined with chemotherapy in the first-line setting, an improvement was seen in survival rates in patients with advanced squamous non–small cell lung cancer (NSCLC), according to updated overall survival (OS) findings from the phase 3 CameL-sq trial (NCT03668496).1 In the trial, a 24-month OS rate of 53.4% was achieved in patients treated with camrelizumab and chemotherapy compared with 35.0% in those treated with chemo- therapy and placebo. At 36 months, the OS rates were 42.8% and 23.7%, respectively (TABLE1).
“These data further support camrelizumab plus chemo[therapy] as a standard first-line treatment option for advanced squamous non–small cell lung cancer,” lead study author Caicun Zhou, MD, PhD, said when presenting prolonged follow-up results with more than 1 year of additional follow-up at the European Lung Cancer Congress 2022. Zhou is director of the Department of Oncology, Shanghai Pulmonary Hospital; director of Cancer Institute of Tongji University Medical School; and chairman of the Oncology Department of Tongji University in China.
The randomized, double-blind trial enrolled patients with pathologically confirmed stage IIIB to IV squamous NSCLC who had not received prior systemic treatment and had an ECOG performance status of 0 or 1. Patients (n = 390) were randomly assigned equally between the camrelizumab and placebo arms and stratified by sex, smoking history, and liver or brain metastasis.
In the investigational arm, patients received 200 mg of camrelizumab, carboplatin area under the curve 5, and 175 mg/m2 of paclitaxel on day 1 of every 3-week cycle for 4 to 6 cycles, depending on the discretion of the investigator. Treatment was then followed by camrelizumab monotherapy at 200 mg for up to 2 years or until disease progression or unacceptable toxicity. Patients assigned to the placebo arm were allowed to cross over to receive camrelizumab at the time of disease progression.
The primary end point was progression-free survival by blinded independent review committee, and secondary end points included OS, objective response rate, duration of response, disease control rate, and safety.
In the primary analysis of the trial with a data cutoff date of November 6, 2020, the combination with camrelizumab showed a significant improvement in progression-free survival compared with chemotherapy alone at 8.5 months vs 4.9 months, respectively (HR, 0.37; 95% CI, 0.29-0.47; P < .0001).2
The presentation included results of follow-up of 23.7 months in the camrelizumab arm and 15.2 months in the placebo arm. As of the updated data cutoff on January 31, 2022, 6 patients were still receiving treatment in the camrelizumab arm and 1 in the placebo arm.1
Baseline characteristics were balanced between the 2 arms. Patients were aged 34 to 74 years, and more than 90% in each arm were male. The majority of patients had a smoking history of at least 400 cigarette-years (82%), ECOG performance status of 1 (79%), and stage IV disease (72%). Twelve percent of patients had either liver or brain metastasis. Approximately half of patients (48%) had positive PD-L1 expression by tumor proportion score, and 21% had a tumor proportion score of at least 50%.
Median OS was 27.4 months (95% CI, 22.1-not reached [NR]) with camrelizumab and chemotherapy compared with 15.5 months (95% CI, 13.4-18.4) with placebo and chemotherapy (HR, 0.57; 95% CI, 0.44-0.74; log-rank 1-sided P <.0001).
Zhou noted that approximately 56% of patients in the placebo arm crossed over to receive camrelizumab. When OS was adjusted for crossover, the median OS was 27.4 months (95% CI, 22.1-NR) with camrelizumab vs 12.4 months (95% CI, 10.9-14.5) without (HR, 0.41; 95% CI, 0.30-0.56; log- rank 1-sided P <.0001).
Patients received a median of 12 cycles of treatment in the camrelizumab arm (range, 1-32) and 7 cycles (range, 1-26) in the placebo arm.
Treatment-emergent adverse events (AEs) were reported in all but 1 patient. In the camrelizumab arm, grade 3/4 AEs were observed in 81.9% of patients vs in 75.0% of the placebo arm. Treatment-emergent AEs led to death in 10.4% and 13.8% of patients in the camrelizumab and placebo arms, respectively.
Grade 3/4 treatment-related AEs were reported in 74.1% of patients in the camrelizumab arm and in 71.4% of patients in the placebo arm. These events led to death in 3.1% and 1.5% of the camrelizumab and placebo arms, respectively.
Immune-related AEs were observed in 77.2% of patients in the investigational arm vs 20.4% of the control arm.
“The majority of events were due to chemotherapy,” Zhou said. The most common treatment-related AEs in the camrelizumab arm were white blood cell count decrease, neutrophil count decrease, anemia, platelet count decrease, reactive capillary endothelial proliferation, and alopecia. Reactive capillary endothelial proliferation was rarely seen in the placebo arm, and there were no grade 3 or higher events.
REFERENCES:
1. Zhou C, Cheng Y, Chen J, et al. First-line camrelizumab plus car- boplatin and paclitaxel for advanced squamous non-small-cell lung cancer: updated overall survival results from the phase 3 CameL-sq trial. Ann Oncol. 2022;33(suppl 2):S27-S70. doi:10.1016/annonc/ annonc856
2. Ren S, Chen J, Xu X, et al; CameL-sq Study Group. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-Sq): a phase 3 trial. J Thorac Oncol. 2022;17(4):544-557. doi:10.1016/j.jtho.2021.11.018
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More




