Clinical Trial Matching Service in Hematologic Cancers Mitigates Barriers to Enrollment
A national, telephone-based nurse navigator-led service model that aimed to reduce barriers to clinical trial participation among patients with a blood cancer and identified patient demographic and clinical characteristics associated with enrollment was shown to mitigate modifiable barriers to trial enrollment.
A national, telephone-based nurse navigator-led service model that aimed to reduce barriers to clinical trial participation among patients with a blood cancer and identified patient demographic and clinical characteristics associated with enrollment was shown to mitigate modifiable barriers to trial enrollment, according to a study published in the JCO Oncology Practice.1
Patients with Medicaid were less likely to enroll in clinical trials than those with private or commercial insurance (adjusted odds ratio, 0.054; 95% CI, 0.003-0.900), and patients in treatment or undergoing maintenance were less likely to enroll than those relapsed or refractory to most recent therapy (adjusted odds ratio, 0.312; 95% CI, 0.139-0.702).
Nurse navigators from The Leukemia & Lymphoma Society’s Clinical Trial Support Center (CTSC) assisted patients with a blood cancer and their oncologists by identifying all appropriate trials based on clinical data and patient preference. They also facilitated informed and shared decision-making and minimized enrollment barriers for 906 patients from October 2017 to October 2019.
Among patients with a blood cancer (n = 750), enrollment was 16.1%. Among a subgroup who had a trial search with a known enrollment outcome (n = 537), the enrollment rate was 22.5%.
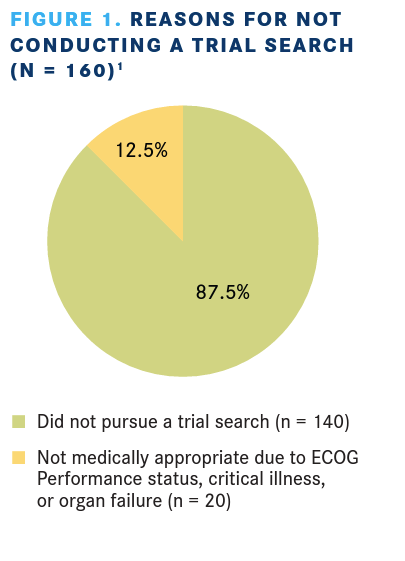
Among patients with a blood cancer, 590 patients or caregivers had a clinical trial search conducted and 160 chose not to. Reasons for not having a trial search conducted are listed in FIGURE 1.1
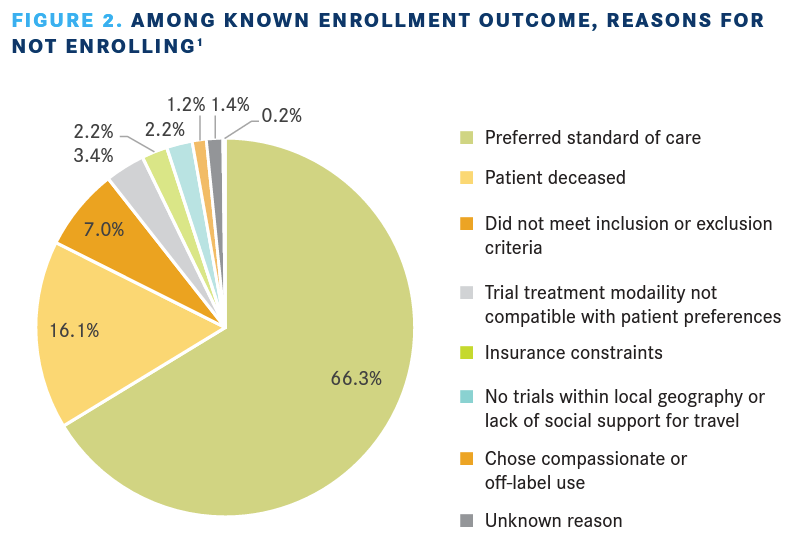
For patients who chose to have a search conducted, 537 patients had a known enrollment outcome and 53 were to follow up after the search. Among the known enrollment outcome group, 121 enrolled in a trial with the help of CTSC and 416 chose not to enroll. Reasons given for not enrolling are provided in FIGURE 2.1
In this subpopulation of patients with known enrollment outcome, the average number of phone and email interactions with a CTSC nurse navigator and any patient or caregiver was 18.1, with the majority of these interactions involving patients or caregivers (80.9%), clinical trial research staff (16.3%), and treating health care providers (2.3%). The average number of interactions for patients who enrolled in a clinical trial was 25.0; for those who did not enroll, the average number of interactions was 16.0.
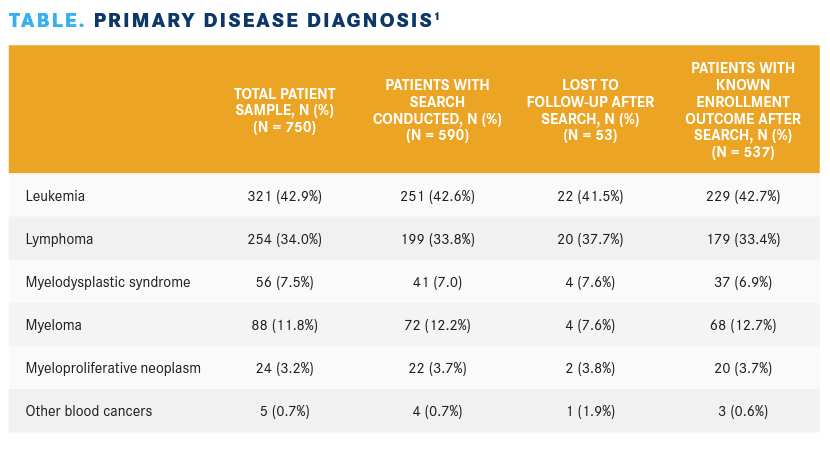
Demographic and clinical information was collected at the commencement of initial contact and included insurance type, treatment status, and ECOG performance status. Primary disease diagnosis is listed in the TABLE.
The investigators noted that the CTSC provided services to and conducted searches for patients who were relapsed or refractory to their most recent treatment; findings indicate that this population was more likely to enroll onto a trial than those undergoing active treatment or maintenance. No significant difference in rate of enrollment between patients seeking first-line treatment and those who were relapsed or refractory were observed. Collectively, the findings point to the benefit of offering clinical trial information earlier in the course of treatment for those patients who are interested.
The nurse navigators reduced patient-level barriers to trial enrollment by providing education that addresses fears and dispels myths. They supplemented site-of-care capacity by providing detailed information about trial status, eligibility, and the trial referral process beyond what may be available on government websites such as ClinicalTrials. gov. They encouraged the patient to take search results to their oncologist for conversation and helped the patient create a list of questions to ask, which facilitates patient-provider communication and shared decision-making. They connected patients to resources that assist with travel, food, and lodging costs, and insurance coverage and appeal. Even after addressing these modifiable barriers, preference for standard of care was the primary driver of nonenrollment; based on nurse navigators’ experience, this was often because of provider recommendation and/or the known effectiveness of an approved therapy.
The investigators noted that the population served by CTSC was predominantly White or Caucasian and as a result, the fi ndings may not represent the experiences of subgroups whose primary language is not English and who have varying cultural preferences and circumstances.
In conclusion, findings demonstrate that a service that matches clinical trials with patients may help to mitigate clinical trial barriers encountered by patients and health care providers that can be replicated across cancer settings.
REFERENCE:
1. Sae-Hau M, Disare K, Michaels M, et al. Overcoming barriers to clinical trial participation: outcomes of a national clinical trial matching and navigation service for patients with a blood cancer. JCO Oncol Pract. Published online June 2, 2021. doi:10.1200/OP.20.01068
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More