As CLL Approaches Expand, Sequencing Questions Arise
Doctors and patients who can stick with a regimen despite relatively minor adverse event will ultimately have more options later in the treatment journey, which could then lead to better long-term outcomes in chronic lymphocytic leukemia.
Nicole Lamanna, MD

A decade ago, a diagnosis of chronic lymphocytic leukemia (CLL) came with very few therapeutic options. “Chemoimmunotherapy was the standard of care,” Nicole Lamanna, MD, associate clinical professor of medicine in the Hematologic Malignancies Section of the Hematology/Oncology Division at Columbia University Herbert Irving Comprehensive Cancer Center in New York, New York, said in an interview with Targeted Therapies in Oncology™.
The only question was whether the patient was young and fit enough to be a candidate for stem cell transplant. Most patients were not, said Anthony Mato, MD, MSCE, director of the CLL Program at Memorial Sloan Kettering Cancer Center in New York, New York. “And they ended up dying of refractory disease or transformation of disease,” Mato said during an interview with Targeted Therapies in Oncology™
Anthony Mato, MD, MSCE

However, a lot can change in a decade. In the case of CLL, the change began in February 2014, with the FDA’s accelerated approval of ibrutinib (Imbruvica) to treat CLL in patients who had received at least 1 prior therapy.1 “That drastically changed things,” Lamanna said.
Ibrutinib was the first Bruton tyrosine kinase (BTK) inhibitor to be granted FDA approval for CLL in November 2013.1 Later, in the RESONATE trial (NCT01578707), the therapy had a 6-month progression-free survival (PFS) rate of 88% and a 12-month overall survival (OS) rate of 90% in previously treated patients with CLL. In this trial, the control group received ofatumumab (Arzerra), and at 12 months, the OS rate was 81% and the PFS was 8.1 months.2
In an accompanying editorial in the New England Journal of Medicine, Robin Foà, MD, professor of hematology and head of the Division of Hematology at the Sapienza University of Rome in Italy, asserted that oncologists were “witnessing a new mechanism-driven era” associated with “the development of compounds capable of targeting the B-cell receptor signaling pathway” in the treatment of CLL.3 That assertion has stood the test of time.
The 8 years since that publication have seen a steady pipeline of new targeted therapies for CLL. The result is that clinicians and patients now have a vastly different range of therapeutic options to choose from. However, with those options have come new questions about how best to choose, sequence, and combine those agents to achieve the best long-term results. Experts such as Lamanna and Mato say the answers to those questions will only come with signifi cantly more research.
A Wave of Approvals Ibrutinib, which was approved as a first-line therapy in 2016, was the first targeted therapy approved for CLL,4 but it was not the last. That same year, the FDA approved idelalisib (Zydelig), an inhibitor of the delta isoform of PI3K, in combination with rituximab (Rituxan) for use in patients with relapsed CLL.5 In 2018, duvelisib (Copiktra) gained FDA approval.6
Meanwhile in 2016, the agency approved venetoclax (Venclexta), initially for patients with 17p deletions whose cancers had progressed after previous treatment with at least 1 other therapy. The drug targets BCL2, which is a protein that promotes tumor cell survival.7 That approval was later expanded to include patients without 17p deletions.8 In 2019, the drug was approved as a first-line CLL therapy in combination with the anti-CD20 monoclonal antibody, obinutuzumab (Gazyva).9
Even as PI3K and BCL2 inhibitors were beginning to hit the market, drug developers were working on second-generation BTK inhibitors, including acalabrutinib (Calquence), which was approved for CLL treatment in 2019,10 and zanubrutinib (Brukinsa), which Lamanna said is likely to gain FDA approval later this year.
The days of chemoimmunotherapy as the major treatment for CLL are over, Lamanna said. “Chemoimmunotherapy is not used very often, if at all,” she said. “Now [oncologists choose] between a BTK inhibitor or venetoclax [plus] obinutuzumab in [the] front line.”
Evolution of BTK Inhibitors
Although BTK inhibition has proven to be a major development in the treatment of CLL, this class of drugs carries a signifi cant risk of adverse events (AEs) and treatment resistance. Newer BTK inhibitors are more selective and have improved the safety profi les for patients with comorbidities.
For instance, results from the phase 3 ELEVATE-RR trial (NCT02477696) published in 2021 showed acalabrutinib had a lower risk of cardiovascular AEs compared with ibrutinib.11 Interim results from the ALPINE study (NCT03734016) showed zanubrutinib had a signifi cantly better overall response rate (ORR) compared with ibrutinib (78.3% vs 62.5%; P = .0006) and led to lower rates of atrial fi brillation or flutter.12
Lamanna noted that historically, patients with significant cardiovascular issues would be considered poor candidates for BTK inhibitors, but that is not necessarily the case anymore. “For somebody who had bad hypertension, bad cardiac issues, and was on anticoagulation, we would avoid ibrutinib and go to venetoclax,” she said. “Now with the second generation, you can challenge that a little. It [depends] on how severe their cardiac issues are.” Lamanna also said the decision can in part depend on the physician’s comfort level with the different therapeutic options.
Mato said comorbidities and fi tness are important to consider when deciding on a treatment regimen. The molecular genetic profile of the malignancy is somewhat less important than it used to be, he said, as BTK inhibitors and venetoclax are “equally useful” options for patients who are standard or high risk. Another factor to consider is patient preference, according to Mato. “The limiting factor of the BTK inhibitors is that they’re continuous therapies,” he said. “People tend to want to stop at some point.”
Noncovalent BTK Inhibitors in the Pipeline
The evolution of BTK inhibition is continuing with noncovalent BTK inhibitors, such as pirtobrutinib and nemtabrutinib. Unlike earlier BTK inhibitors, they do not bind covalently to C481 of the BTK protein, making them a meaningful option, even for patients with acquired resistance to earliergeneration agents.13
In phase 1/2 data published in 2021, investigators, including Lamanna and Mato, found that pirtobrutinib produced strong ORR results, even in patients who had previously been treated with BTK inhibitors. ORRs of 67%, 52%, and 71% were found in patients with previous resistance to covalent BTK inhibitors, previous intolerance to BTK inhibitors, and BTK C481 mutations, respectively.14 Those data suggest pirtobrutinib may be another treatment option for patients with BTK C481 mutations who have become resistant to ibrutinib.
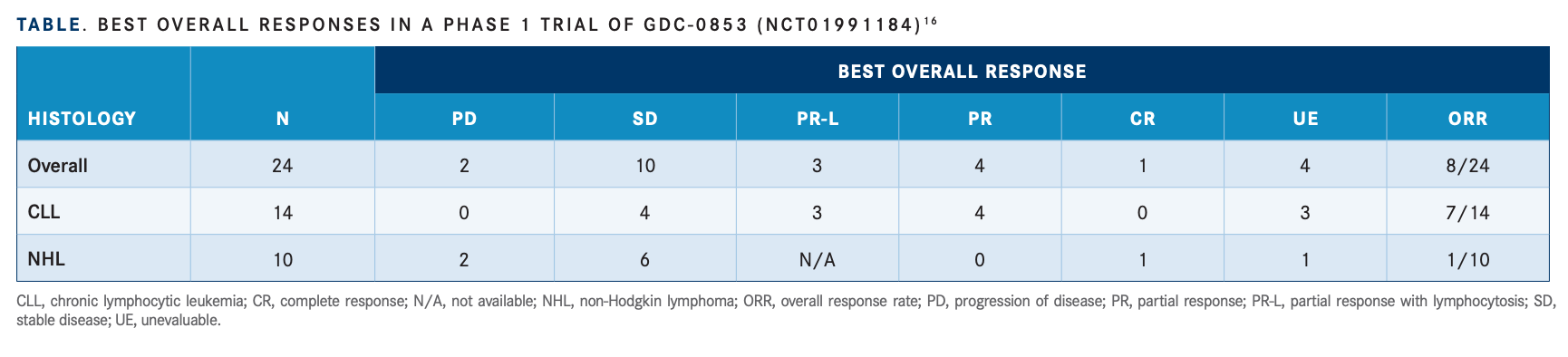
Trials are also under way for other noncovalent BTK inhibitors, including nemtabrutinib. In results presented the European Hematology Association 2022 Congress, investigators found the therapy had an acceptable safety profi le and an ORR of 57.9% in heavily pretreated patients.15 Another drug, fenebrutinib, was the subject of a recently completed phase 1 study (NCT01991184).16 Twenty-four patients were enrolled in this study and 8 patients had an ORR. Best responses included 3 patients with partial response with lymphocytosis, 4 with a partial response, and 1 with a complete response (TABLE16).
Meanwhile, Sunesis Pharmaceuticals, Inc, terminated a phase 1b trial of vecabrutinib, yet another noncovalent BTK inhibitor. In that case, investigators found the drug was well tolerated but did not appear to have sufficient evidence of antitumor activity.17
For Lamanna, the big picture is that these third-generation BTK inhibitors have the potential to further expand the treatment landscape, thereby increasing the range of therapeutic strategies for patients and their physicians. “The point is we’ll have [more drugs] in our armamentarium,” she said. “Of course, our goal would be to cure CLL, but if we can’t cure CLL, you’re always thinking, ‘How do I keep these patients going? How do I go from one drug to another drug and really make it a chronic disease?’”
Combining Targeted Therapies The new CLL therapies have also created the opportunity for new fixed-duration combination regimens, including those studied in the GLOW and CAPTIVATE trials. In December 2021, results from the phase 3 GLOW trial (NCT03462719) showed that combining ibrutinib with venetoclax led to fewer fi rst-year relapses in treatment-naïve patients who were elderly or unfit, compared with chlorambucil (Leukeran) plus obinutuzumab. At 30 months, patients in the ibrutinib plus venetoclax cohort had a PFS rate of 80.5% compared with 35.8% for patients receiving chlorambucil plus obinutuzumab. The trial did not include patients with 17p deletions or known TP53 mutations.18
Findings from the CAPTIVATE trial (NCT02910583) of ibrutinib plus venetoclax have also been promising.19 This trial produced an ORR of 96% in treatment-naïve patients who were 70 years or younger at a 3-year follow-up. That study was notable in part because the response data were consistent, even for patients who were highrisk and including those with 17p deletions, TP53 mutations, unmutated IGHV, and/or complex karyotypes.
Beyond evaluating the efficacy of the treatment combination, this study is also important because it could help investigators better understand the role minimal residual disease (MRD) can play in tracking patient responses to therapy. Lamanna noted that CAPTIVATE is designed so that after a prerandomization phase, patients who are MRD positive will continue treatment with ibrutinib either alone or combined with venetoclax. Those who have confi rmed undetectable MRD will continue with ibrutinib or be switched to placebo. The results could help oncologists better understand whether and how MRD can be used to guide therapy.
“Patients and specialists want to use MRD to guide exposure,” Mato said. “There has not been a lot of use of MRD, but I think people are ready to go in that direction. I really do.” He also added that, so far, no CLL therapies have been approved based on trials with an MRD end point; however, an MRD assay has been approved.
Another combination therapy, venetoclax plus rituximab, was the subject of the MURANO trial (NCT02005471), which found superior OS and PFS at 5 years compared with bendamustine (Treanda) plus rituximab. The estimated 5-year OS was 82.1% for the venetoclax regimen vs 62.2% for the bendamustine regimen.20
In addition, a phase 1 trial (NCT03328273) evaluating the second-generation BTK inhibitor acalabrutinib plus the ataxia telangiectasia and RAD3-related inhibitor ceralasertib (also known as AZD6738) is currently ongoing.21
Retreatment Options
Investigators are also examining how best to sequence therapies and what strategy to take when a patient experiences relapse or needs to discontinue a medication. Lamanna said patients who start with a BTK inhibitor can be easily retreated with venetoclax and rituximab or venetoclax plus a monoclonal antibody. “There’s no doubt that we have very good data that [show] the venetoclax-based regimens can salvage patients who either have intolerance to a BTK [inhibitor] or who have progressed,” she said. In such cases, patients can take fixed-duration venetoclax-based regimens, and if all goes well, they may be able to completely go off therapy for months or even years.
A 2020 study showed that, in cases where patients discontinued venetoclax, BTK inhibitors were the most effective choice for their next therapy, provided the patient was either BTK inhibitor naïve or had previously responded to a BTK inhibitor.22 Yet another study found that patients with venetoclax-based regimens can benefit from retreatment with venetoclax following remission, particularly if they achieved a complete response from their initial therapy.23
Lamanna said the optimal treatment remains to be determined for patients who receive a doublet or triplet combination and eventually relapse. “Hopefully some of these studies will help tell us,” she said. The trials could also answer questions about whether fixed-duration therapies are appropriate for all patients, including patients who are high-risk, according to Lamanna.
The Role of CAR T-Cell Therapy
Another potential option for patients is cellular therapies, such as lisocabtagene maraleucel (Breyanzi), which was approved in 2021 in combination with ibrutinib to treat relapsed or refractory large B-cell lymphoma. Lisocabtagene-maraleucel is an autologous, CD19-directed chimeric antigen receptor (CAR) T-cell therapy.24
Lamanna noted that the idea of using CAR T cells to treat CLL has been around for more than a decade, but the AEs associated with the therapy required a signifi cant learning curve. However, she noted that significant progress has been made in terms of curbing AEs. “That’s the good news,” she said. The bad news is that CAR T has yet to achieve wide adoption within the CLL oncology community because of the steady stream of other therapies that have become available, Lamanna explained.
“It’s still being relegated a little bit to specialty places [that] could do CAR T,” Lamanna said. She believes CAR T-cell therapy will eventually be approved for CLL, but in a crowded field of therapies, she does not expect it to become a prominent component of CLL care.
Mato said he believes cellular therapies will have a role in the future of CLL therapy. “It’s a question about what line [of therapy],” he said. “Current patients are referred after they’re out of options. Sometimes that probably limits the success of the cellular therapy, because not only do you need to be able to harvest T cells, but you [also] need to have functional T cells.” Mato also said it will be important to have studies that examine the ideal candidates and sequence to offer CAR T-cell therapy.
Many Questions Remain
The need for more research is a major theme in Mato’s assessment of the current CLL treatment picture. Although he is excited about the new therapies, he also thinks drug developers need to do a better job of carrying out relevant trials that will help oncologists better understand which of the new therapies work best in which situations. “The limitations are that they’re never compared [with] one another,” he said.
Most of the studies still compare the newer therapies with older, less-relevant regimens. Mato said a more helpful approach would be to test these new targeted therapies against other targeted therapies. “What we really need to move the fi eld forward are headto-head comparisons of relevant classes of drugs, and we need studies that allow us to understand sequencing,” he said.
Part of the reason such studies are rare is that drug developers are reticent to do competitions among “winners,” because the results might mean their product goes from being a go-to therapy to being a therapy that is less sought after, Mato explained. Thus, it is hard to fi nd industry support for the kinds of large-scale, head-to-head studies that are necessary. “If you’re not going to have that support, then the ability to fund a meaningful randomized clinical trial is really hard to come up with,” he said
On a smaller scale, Lamanna said there is also a need for clinicians to expand the knowledge of these new therapies by being strategic about how they use them. She cautioned that clinicians should not be too quick to change from one therapy to the next when patients experience AEs. Although patient status and toxicities may warrant a change in therapy, she said not every AE requires stopping the drug. “Sometimes a little diarrhea [or] nausea—you can work through them,” she said, noting that careful patient counseling ahead of time and thoughtful management of AEs during treatment can allow patients to continue on a therapy in many cases.
Doctors and patients who can stick with a regimen despite relatively minor AEs will ultimately have more options later in the treatment journey, which could then lead to better long-term outcomes. “It takes more time and effort, but it’s worth it, because these drugs are going to be the mainstay of our treatment for CLL for quite some time,” she said.
REFERENCES:
1. Imbruvica (ibrutinib) now approved in the U.S. for patients with chronic lymphocytic leukemia who have received at least one prior therapy. News release. Johnson & Johnson; February 4, 2014. Accessed August 4, 2022. https://bit.ly/3SpAJPe
2. Byrd JC, Brown JR, O’Brien S, et al; RESONATE Investigators. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213-223. doi:10.1056/NEJMoa1400376
3. Foà R. Changes in the treatment landscape for chronic lymphoid leukemia. N Engl J Med. 2014;371(3):273-274. doi:10.1056/NEJMe1405766
4. Imbruvica (ibrutinib) approved by the U.S. FDA for the first-line treatment of chronic lymphocytic leukemia. News release. AbbVie; March 4, 2016. Accessed August 4, 2022. https://bit.ly/3byT9fQ
5. U.S. Food and Drug Administration approves Gilead’s Zydelig (idelalisib) for relapsed chronic lymphocytic leukemia, follicular lymphoma and small lymphocytic lymphoma. News release. Gilead; July 23, 2014. Accessed August 4, 2022. https://bit.ly/3zYfDAf
6. Duvelisib (Copiktra, Verastem, Inc.) for adult patients with relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). News release. FDA; September 24, 2018. Accessed August 4, 2022. https://bit.ly/2Zmrl2f
7. FDA approves new drug for chronic lymphocytic leukemia in patients with a specific chromosomal abnormality. News release. FDA; April 11, 2016. Accessed August 4, 2022. https://bit.ly/3zUXVxA
8. FDA approves new treatment option for previously treated chronic lymphocytic leukemia. News release. Genentech; June 8, 2018. Accessed August 4, 2022. https://bit.ly/2l0fuVD
9. AbbVie announces US FDA approval of Venclexta (venetoclax) as a chemotherapy-free combination regimen for previously untreated chronic lymphocytic leukemia patients. News release. AbbVie; May 15, 2019. Accessed August 4, 2022. https://bit.ly/2OGvJJp
10. Project Orbis: FDA approves acalabrutinib for CLL and SLL. News release. FDA; November 21, 2019. Accessed August 4, 2022. https://bit.ly/3BCs6ee
11. Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021;39(31):3441-3452. doi:10.1200/JCO.21.01210
12. Hillmen P, Eichhorst B, Brown JR, et al. First interim analysis of ALPINE study: results of a phase 3 randomized study of zanubrutinib vs ibrutinib in patients with relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma. Presented at: European Hematology Association 2021 Congress; June 9-17, 2021; virtual. Abstract LB190.
13. Lewis KL, Cheah CY. Non-covalent BTK inhibitors-the new BTKids on the block for B-cell malignancies. J Pers Med. 2021;11(8):764. doi:10.3390/jpm11080764
14. Mato AR, Shah NN, Jurczak W, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet. 2021;397(10277):892-901. doi:10.1016/S0140-6736(21)00224-5
15. Woyach J, Flinn IW, Awan FT, et al. Nemtabrutinib (MK-1026), a non-covalent inhibitor of wild-type and C481S mutated Bruton tyrosine kinase for B-cell malignancies: efficacy and safety of the phase 2 dose-expansion BELLWAVE-001 study. Presented at: European Hematology Association 2022 Congress; June 9-12, 2022; Vienna, Austria. Abstract P682. https://bit.ly/3HDw2fC
16. Byrd JC, Smith S, Wagner-Johnston N, et al. First-in-human phase 1 study of the BTK inhibitor GDC-0853 in relapsed or refractory B-cell NHL and CLL. Oncotarget. 2018;9(16):13023-13035. doi:10.18632/oncotarget.24310
17. Allan JN, Patel K, Mato AR, et al. Ongoing results of a phase 1b/2 dose-escalation and cohort-expansion study of the selective, noncovalent, reversible Bruton’s tyrosine kinase inhibitor, vecabrutinib, in B-cell malignancies. Blood. 2019;134(suppl 1):3041. doi:10.1182/blood-2019-126286
18. Kater A, Owen C, Moreno C, et al Fixed-duration ibrutinib and venetoclax (I+V) versus chlorambucil plus obinutuzumab (CLB+O) for first-line (1L) chronic lymphocytic leukemia (CLL): primary analysis of the phase 3 GLOW study. Poster presented at: European Hematology Association 2021 Congress; June 9-17, 2021; virtual.
19. Wierda WG, Barr PM, Siddiqi T, et al. Fixed-duration (FD) ibrutinib (I) + venetoclax (V) for first-line (1L) treatment (tx) of chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL): three-year follow-up from the FD cohort of the phase 2 CAPTIVATE study. J Clin Oncol. 2022;40(suppl 16):7519. doi:10.1200/JCO.2022.40.16_suppl.7519
20. Kater AP, Kipps TJ, Eichhorst, B, et al. Five-year analysis of Murano study demonstrates enduring undetectable minimal residual disease (uMRD) in a subset of relapsed/refractory chronic lymphocytic leukemia (R/R CLL) patients (Pts) following fixed-duration venetoclax-rituximab (VenR) therapy (Tx). Presented at: 62nd American Society of Hematology Annual Meeting and Exposition; December 5-8, 2020; virtual. Abstract 125. Accessed August 1, 2022. https://bit.ly/3d8OCRu
21. Jurczak W, Elmusharaf N, Fox CP, et al. Phase 1/2 results of ceralasertib (Cerala) as monotherapy or in combination with acalabrutinib (Acala) in high-risk relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL). Presented at: American Association for Cancer Research Annual Meeting 2022; April 8-13, 2022; New Orleans, LA. Abstract CT532. Accessed April 8, 2022. https://bit.ly/3JvBzpz
22. Mato AR, Roeker LE, Jacobs R, et al. Assessment of the efficacy of therapies following venetoclax discontinuation in CLL reveals BTK inhibition as an effective strategy. Clin Cancer Res. 2020;26(14):3589-3596. doi:10.1158/1078-0432.CCR-19-3815
23. Thompson MC, Allan JN, Sail K, et al. Venetoclax re-treatment of chronic lymphocytic leukemia (CLL) patients after a previous venetoclax-based regimen. Presented at: 62nd American Society of Hematology Annual Meeting and Exposition; December 5-8, 2020; virtual. Abstract 3136. Accessed August 1, 2022. https://bit.ly/3OZwzKT
24. U.S. FDA approves Bristol Myers Squibb’s CAR T cell therapy Breyanzi for relapsed or refractory large B-cell lymphoma after one prior therapy. News release. Bristol Myers Squibb; June 24, 2022. Accessed August 12, 2022. https://bit.ly/3C0lEOa
