ALK-Rearranged NSCLC Brain Metastasis Study Demonstrates Extended Survival
A paper presenting the results of a retrospective study of patients with ALK-rearranged NSCLC hypothesized that prognosis for patients with NSCLC and brain metastases could be further identified through molecular subtyping.
NSCLC

Joseph N. Contessa MD, PhD
A paper presenting the results of a retrospective study of patients with anaplastic lymphoma kinase (ALK)-rearranged nonsmall-cell lung cancer (NSCLC) and brain metastasis has been published in a recent edition of theJournal of Clinical Oncology.1The study was conceived and designed by Kimberly L. Johung, MD, PhD, assistant professor of therapeutic radiology, and director gastrointestinal radiotherapy program along with Joseph N. Contessa MD, PhD, associate professor of therapeutic radiology and of pharmacology at the Yale Cancer Center in New Haven. They hypothesized that prognosis for patients with NSCLC and brain metastases could be further identified through molecular subtyping. This subtyping would identify prognostic factors to enable tailored treatments.
“There is very little data on the optimal management of patients withALKtranslocations and brain metastases in the literature. Yale is a large referral center for patients with NSCLC brain metastases, and we began to see these patients and to ask the question, ‘What is the best way to manage and treat this population?’” said Contessa.
The authors write that although crizotinib-treated patients (approved for the treatment of patients with metastatic NSCLC whose tumors areALK-positive as detected by an FDA-approved test),2experience prolonged progression-free survival (PFS), brain metastases, which may progress, are common in this population.3,4One reason for this may be poor penetration of crizotinib into the brain,5bringing to the forefront the use of whole brain radiotherapy (WBRT), stereotactic radiosurgery (SRS), or surgical resection; used alone, in combination, or sequentially at sites of oligoprogression to improve outcomes.1
The Patients
This retrospective study examined the medical data from 90 patients withALK-rearranged NSCLC diagnosed between 2007 and 2014. A total of 84 of the 90 patients had been treated with radiation therapy (RT) for brain metastases, and 86 had received treatment with tyrosine kinase inhibitors (TKIs).1In an interview withTargeted Oncology, senior author Joseph N. Contessa, MD, PhD cited the most rigorous aspect of the study was that, “The patient data was accrued from six academic cancer centers in the United States.”
The median age of the patients was 52 years (23-80) and the majority were nonsmokers (67%), who were found to have stage IV disease; 30% had brain metastases at diagnosis. Detected by magnetic resonance imaging (MRI), brain metastases developed in 70% of patients at a median of 27 months from diagnosis (2-174 months), and almost 50% of patients were found to have ≥4 metastases upon initial examination. At the time of diagnosis of brain metastases, 69% of patients were found to also have extracranial metastases versus 30% who had brain metastases only.1
At the time of brain metastases development patient Karnofsky Performance Scores (KPS) were high, and the authors concluded that the brain metastases were minimally symptomatic, responsive to steroid treatment, or asymptomatic for the majority of patients.1
In total, 84 patients were treated with crizotinib2and 41 with a second-generationALKinhibitors (ceritinib,6n = 21; AP-26113,7n = 16; alectinib,8,9n = 2; X-396,10n = 2). Among the 44 patients who had received prior treatment with anALK-targeted TKI before subsequently presenting with brain metastases, crizotinib was prescribed to 38 patients, and crizotinib followed by a second-generationALKinhibitor prescribed to 6 patients.
Radiation Therapy and Neurosurgical Procedures
SRS was performed in 119 instances in 64 patients, and 45 patients received 48 courses of WBRT, while 16 patients were treated with 21 neurosurgical procedures. Retreatment with RT was common among the cohort; 43 of 84 patients experienced a repeat procedure and 21 of 84 had ≥3 procedures performed. During SRS, a median of two lesions (1-18) were treated per session.1
Comparing SRS and WBRT Contessa said, “WBRT has also been thought of as a way to treat detectable and undetectable (microscopic) disease in the brain while [using] SRS only, treats the disease visible on MRI. One might think that this would be a potential advantage of WBRT, but we find that regardless of SRS or WBRT treatment first (and due to their extended survival), many patients continue to develop brain metastases and need more RT treatment, typically with a second course of SRS.” Seventeen patients underwent 22 craniotomies. Gross tumor was resected in 15 individuals, and necrosis in 5. The authors wrote that, “On average, for each year of life after diagnosis of brain metastasis, 1.4 brain treatments were performed in year 1, 0.6 in year 2, 0.7 in year 3, and 0.5 in year 4 for those patients still alive and with follow-up.”1
Survival
The median overall survival (OS) following diagnosis of brain metastasis was 49.5 months (0.2-82.2; 95% CI, 29 to not reached), and Kaplan Meier estimates of 1- and 2-year OS were 72% and 66%, respectively.1When asked if these results were unexpected, Contessa said, “Yes. Patients withALKtranslocation and brain metastasis have a median survival of approximately 4 years. This is the longest survival for any subgroup of patients that develop brain metastasis from cancer.”
The authors noted that even using the most favorable graded prognostic assessment (GPA) (age ≤50 years, KPS ≥90, no ECM, single brain metastasis) as an indicator of prognosis yields only an estimated survival of 14.8 months.1,11It clearly is not prognostic for these patients withALK-rearrangements. The data demonstrate that within the realm of present-day TKI treatment and RT,ALKrearrangement can stand alone as a robust predictor for improved survival in patients with NSCLC brain metastases.1
In patients with metastatic disease limited to the brain (median survival not reached) versus patients with ECM and brain metastases, OS was significantly longer (median survival, 32.6 months,P= .003). For patients with 1 brain metastasis versus patients with >1 metastasis there was no difference in survival (63.3 versus 49.5 months;P= .633).1
In 49% of patients, the TKI therapy began before the development of brain metastases, and these patients had a significantly shorter OS than patients who began TKI therapy after the development of metastases (Figure 1).1
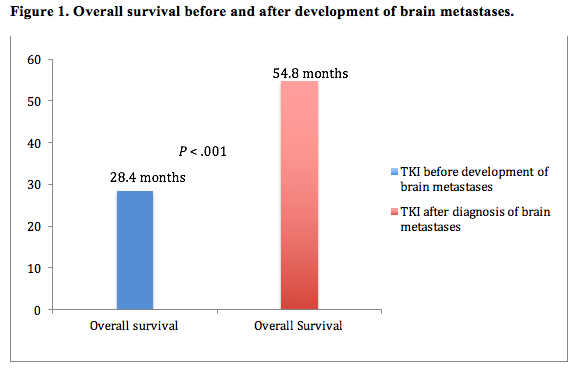
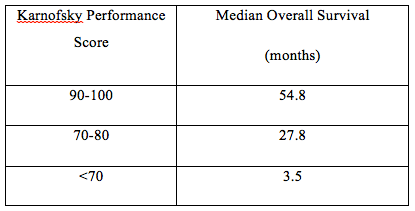
“Patients that received a TKI and then develop brain metastasis have likely developed resistance to that TKI,” Contessa said. “This means worse control of systemic disease and reduced survival. Patients that are TKI naïve will have better control of their systemic disease with a TKI and likely a longer OS.” There was no difference between these two ngroups of patients in the progression of intracranial brain metastases (Median PFS 11.7 vs 11.9 months respectivelyP= .978). The team also found that OS varied significantly by KPS (Table 1)1
Table 1.Relationship Between Overall Survival and Karnofsky Performance Score (P<.001)
Turning to multivariable analysis, the team identified independent predictors of OS to be:
- treatment naïve with regard toALK-targeted TKIs
- no ECM
- KPS of ≥90, (P<.001,P= .003,P<.001)
Prognosis was not significantly influenced by age, smoking history, number of metastases in the brain (one versus many), or the initial type of RT (SRS vs WBRT).1Referring to SRS and WBRT, Contessa explained, “There was no difference in survival for patients that received WBRT versus SRS first (P= .666). Either form of radiation treatment to the brain can control brain metastases for some time. However, WBRT is associated with more side effects including long terms side effects on cognition and so we recommend SRS.”
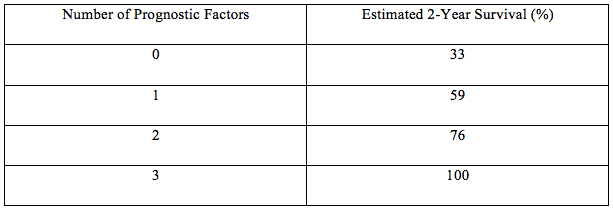
Using the prognostic factors they identified by multivariable analysis, they were able to estimate survival for four patient groups (Table 2).1
Table 2. Estimated Survival for Four Patient Groups
The cause of death was not known for the majority of the patients who died (34). It was attributed to neurologic complications in five patients. Of patients with follow-up, 13/29 had progressive metastatic disease at the time of death, and 10/30 had symptomatic brain metastases.1
Summary
When managed with TKIs and radiotherapy, patients withALK-rearranged NSCLC had prolonged survivallonger than for any subgroups that develop brain metastasis from cancer. In addition repeat treatments to control intracranial disease were evidently of therapeutic value. The study also identified clinical prognostic factors that permit the classification of patients into risk groups—a valuable tool for clinical trials—or for making treatment decisions regarding radiotherapy. The development ofALK-targeted TKIs with enhanced central nervous system (CNS) penetration would also be of value to this population and the authors stated, “Even a modest increase in CNS availability is likely to have a significant impact on this patient population, and evaluations of second-generationALKinhibitors for efficacy in the CNS are currently under way.”1,8,9
Referring to the implications of the study findings for treatment practice, Contessa said, “All patients with lung adenocarcinomas and brain metastasis will need to haveALKtesting performed. We also strongly recommend that these patients be managed with stereotactic radiosurgery when possible instead of whole brain radiation therapy.”
Clinical Pearls
- Patients withALK-rearranged NSCLC and brain metastases have prolonged survivallonger than any other subgroup of patients that develop brain metastasis from cancer.
- This has been achieved using a combination ofALK-targeted TKIs and radiotherapy (as SRS or WBRT).
- Close monitoring and repeat interventions are necessary to maintain disease control.
- The following clinical factors may be used to assign patients into risk groups:
- KPS;
- absence of ECM;
- and no history ofALK-directed TKI therapy before developing brain metastases.
- All patients with lung adenocarcinomas and brain metastasis should have anALKtest.
- Stereotactic radiosurgery should be used if possible instead of whole brain radiation therapy.
- There is a need forALK-targeted TKIs with improved CNS penetration.
References
- Johung KL, Yeh N, Desai NB, et al. Extended survival and prognostic factors for patients withALK-rearranged non-small-cell lung cancer and brain metastasis.J Clin Oncol.2015. pii: JCO.2015.62.0138. PubMed PMID: 26438117. Accessed November 5, 2015.
- Xalkori [prescribing information]. New York, NY: Pfizer Inc; 2015.http://labeling.pfizer.com/showlabeling.aspx?id=676. Accessed November 9, 2015.
- Chun SG, Choe KS, Iyengar P, Yordy JS, Timmerman RD. Isolated central nervous system progression on crizotinib: an achilles heel of non-small cell lung cancer with EML4-ALK translocation?Cancer Biol Ther.2012;13(14):1376-1383.
- Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer.J Thorac Oncol. 2012;7(12):1807-1814.
- Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib.J Clin Oncol. 2011;29(15):e443-445.
- Zykadia [prescribing information] East Hanover, NJ: Novartis Pharmaceuticals Corp. 2105.http://www.pharma.us.novartis.com/product/pi/pdf/zykadia.pdfAccessed November 13, 2015.
- ESMO. ELCC 2015 News. Brigatinib (AP26113) shows intracranial anti-tumour activity in ALK-positive NSCLC patients with brain metastasis following crizotinib.http://www.esmo.org/Conferences/Past-Conferences/ELCC-2015-Lung-Cancer/News-Press-Releases/Brigatinib-AP26113-Shows-Intracranial-Anti-tumour-Activity-in-ALK-positive-NSCLC-patients-with-Brain-Metastasis-Following-Crizotinib. Accessed November 13, 2015.
- Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study.Lancet Oncol. 2014;15(10):1119-1128.
- Gainor JF, Sherman CA, Willoughby K, et al. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib.J Thorac Oncol. 2015;10(2):232-236.
- Horn L, Infante JR, Blumenschein GR et al. A phase I trial of X-396, a novel ALK inhibitor, in patients with advanced solid tumors.J Clin Oncol32:5s, 2014 (suppl; abstr 8030).
- Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases.J Clin Oncol. 2012;30(4):419-425.
