AI Delivers Greater Precision in Oncology Care
In the context of oncology, the use of artificial intelligence/machine learning has enabled the growth of precision medicine, helping clinicians increase efficiency and accuracy, according to Alexander S.Baras, MD, PhD.

As part of the clinical science symposium offered during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago, Illinois, a special session titled “Is There a Ghost in the Machine? Putting Artificial Intelligence to Work” convened experts to explore methods by which artificial intelligence and machine learning allow clinicians more precision in delivering care for patients with cancer.1
In the context of oncology, Alexander S. Baras, MD, PhD, director, pathology informatics, and associate professor of pathology at Johns Hopkins Medicine in Baltimore, Maryland, explained that use of artificial intelligence/machine learning (AI/ML) has enabled the growth of precision medicine, helping clinicians increase efficiency and accuracy.
“I see AI/ML as augmenting, not replacing, humans in how we deliver care to our patients,” Baras said. ML algorithms can perform tasks salient to the management of cancer in patients (ie, screening pathology slides for presence and type of disease, combining genomic profile with biomedical literature to inform treatment options, and monitoring treatment response using cellfree DNA from blood).
To be effective, AI/ML requires good data, but Baras added the caveat that systems and models can still learn under weak supervision.
Not only should data be acceptable, but large amounts are necessary to train the model that can better inform delivery of care. Baras advised being careful about how data are generated and to be aware of the potential for bias, in the context of clinical trials and of routine care. Further, it is essential to enable the data to train models for future applications.
Baras noted that ML techniques have had an impact in oncology. Tools, such as the Oncotype Dx, are multivariate models that use ML techniques to predict recurrence of breast cancer. ML has also improved clinical trial matching by requiring the codification of eligibility criteria.
In the immune-oncology arena, Baras said the success of some immunomodulatory therapeutics have brought the interface of tumor biology and immunology to the forefront. “[ML] techniques have characterized the intrinsic properties of the immune system, such as the immune synapse, where antigens are presented and then recognized by the immune system,” Baras said. ML helps to capture the complexities at that level of biology with the aim of potentially leveraging the data to predict a response to therapy. These techniques can also help investigators understand the relationship between intrinsic molecular properties in space and in time, Baras added.
Baras recalled that similar progress was observed with the introduction of genomics and bioinformatics. He said this technology was soon embraced and has become an essential part of clinical care today. “I think the same thing will happen with the application of [ML] techniques. It’s going to be an augmentation, not a replacement, of our ability to be precise,” Baras said.
Multimodal AI Tool In the next presentation of the symposium, Mack Roach III, MD, a radiation oncologist at UCSF Health in California, explored the potential of generalizing a prostate cancer tool that used multimodal AI (MMAI) deep learning based on digital histopathology and clinical data generated from a series of NRG Oncology phase 3 clinical trials.2
In the study, data were collected from the NRG Biobank (N=5654) and involved 5 clinical trials: RTOG 9202 (NCT00767286), RTOG 9413 (NCT00769548), RTOG-9910 (NCT00005044), RTOG 0126 (NCT00033631), and RTOG-9408 (NCT00002597). The model was developed using a training set, that made up of 80% of the data, and a validation set that made up 20% of the data. Clinical outcomes were biochemical failure, distant metastasis, prostate cancer–specific mortality, and overall survival.
Overall median age was 70 years (range, 66-74) and there were 932 African American patients and 4692 non–African American patients. Thirty patients were of unknown race status. Baseline characteristics were similar for Gleason score; clinical T stage; low-risk, intermediate-risk, or high-risk group; and follow-up time.
Roach said clinical data and digital pathology slides were put into a workflow, cut into segments, and underwent various AI approaches. These segments were eventually recombined and fully connected to generate an AI score.2
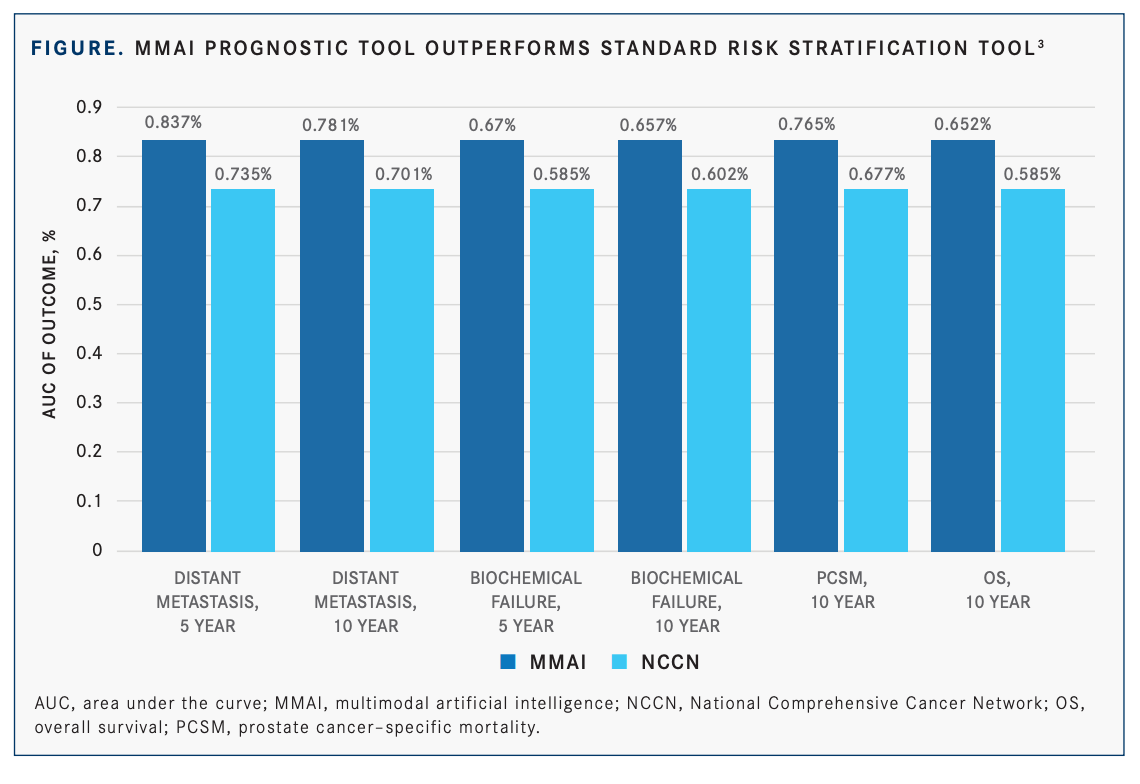
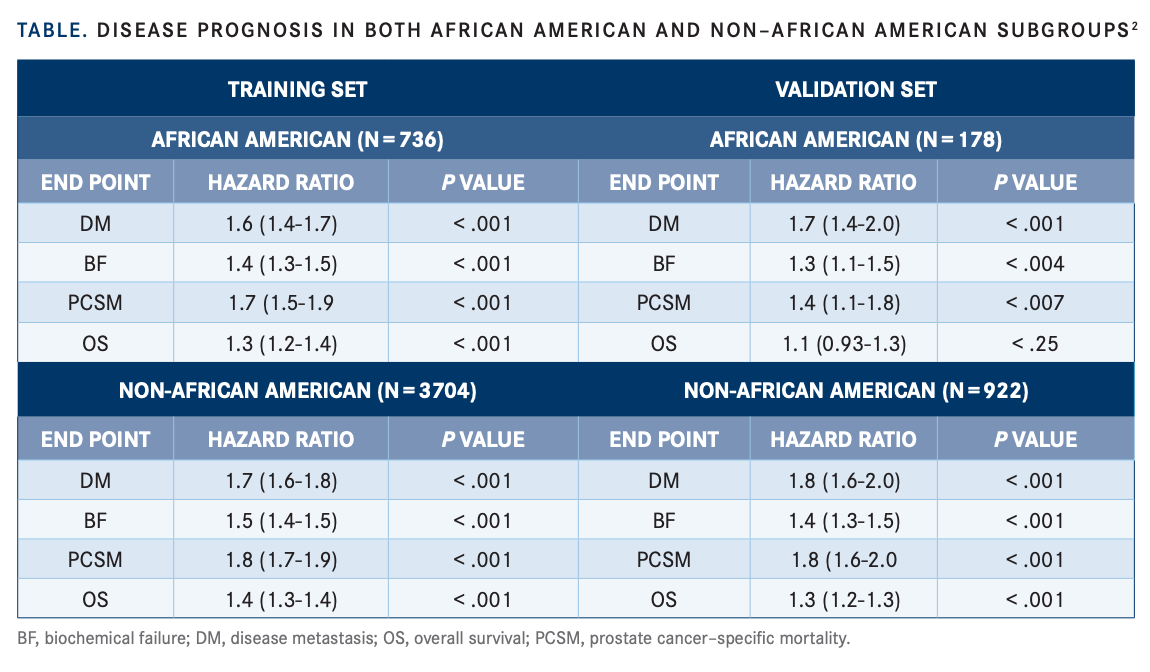
When comparing the MMAI tool outcomes with the National Comprehensive Cancer Network (NCCN), the standard approach, Roach said the MMAI tool is an improvement over what is typically used to make treatment recommendations and to discuss prognosis. The MMAI model outperforms NCCN for all end points: distant metastasis, biochemical failure, prostate cancer–specific mortality, and overall survival (FIGURE).3 Roach added that when comparing prognostic score distributions between the 2 populations, the scores were similar. Turning to the training and validation set, Roach said HR for all the end points for both populations were similar (TABLE2 ).
“The bottom line is that the long-term disease [prognoses] for African Americans and non–African Americans are well predicted when using the AI model. The AI model was very robust for predicting these clinical end points,” Roach said.
Roach emphasized that all biomarkers be applied broadly in the community and that they be studied in all populations, “particularly high-risk populations in order to make sure that disparities and equity are not compromised,” he concluded.
Providing Commentary
Rana R. McKay, MD, an associate professor of medicine at UC San Diego Health in California, noted that prostate cancer is a complex disease that disproportionately affects African American men in the United States. “African American men have a higher incidence of prostate cancer, they are less likely to receive treatment, more likely to experience treatment delays, and less likely to receive high-quality care,” McKay said.
Referring to the study, McKay said the investigators used archival prostate biopsy tissue and prospectively collected clinical data from 5 phase 3 clinical trials of men with prostate cancer who received definitive radiation therapy with or without androgen deprivation therapy.
Most archival specimens were at least 15 years old, and yet it’s remarkable that the model was predictive with the use of these older specimens,” McKay said. “I think understanding the age of the sample affects model performance. This will be important for the future.”
Reviewing the baseline characteristics of the patients, McKay said that most patients (90%) had intermediate-risk and high-risk disease, the median follow-up time was 10 years, and that NRG has historically done an excellent job with accruing studies. She added, “[Although] intraductal carcinoma has recently been associated with negative outcomes, conceivably the model would be able to detect for this and account for it. Further, overlaying genomic classifiers such as Decipher are also considerations for the future.”
She noted that a recent study demonstrated that the Decipher genomic classifier improved the prognostic ability of standard-of-care clinical and pathological variables.4 Nairath et al reviewed the evidence of the Decipher genomic classification tool for men with prostate cancer and performed a systematic review of the available evidence supporting the clinical utility of Decipher, encompassing 42 studies and 30,407 patients.4
The investigators concluded that consistent data are now present from diverse levels of evidence, demonstrating clinical utility of the genomic classifier in prostate cancer. The utility of the genomic classifier is strongest for intermediate-risk prostate cancer and postprostatectomy decision-making.4
“The MMAI model for improved prediction of the NCCN criteria had similar distributions between African American and non–African American subgroups. This work highlights the revolutionary application of AI into clinical practice and overcomes some of the historical limitations of earlier AI,” McKay said.
“Further, the tool learns from patient-level data and annotated histopathology slides. It’s scalable into practice given minimal tissue requirements, low costs, and fast turnaround time,” McKay concluded, noting that national professional organizations, ASCO included, have launched initiatives to bridge further knowledge gaps and promote AI in oncology.
REFERENCES:
1. Baras AS. The future is now: the impact of artificial intelligence in oncology. Presented at: 2022 American Society of Clinical Oncology Annual Meeting; June 6, 2022. Accessed June 9, 2022. https://bit.ly/3xfN9Q1.
2. Roach M III, Zhang J, Esteva A, et al. Prostate cancer risk in African American men evaluated via digital histopathology multi-modal deep learning models developed on NRG Oncology phase III trials. Presented at: 2022 American Society of Clinical Oncology Annual Meeting. Accessed June 9, 2022. https://bit.ly/3xfN9Q1.
3. Esteva A, Feng J, van der Wal D, et al. Prostate cancer therapy personalization via multi-modal deep learning on randomized phase III clinical trials. NPJ Digit Med. 2022;5(1):71. doi:10.1038/s41746-022-00613-w
4. Jairath NK, Dal Pra A, Vince R Jr, et al. A systematic review of the evidence for the Decipher genomic classifier in prostate cancer. Eur Urol. 2021;79(3):374-383. doi:10.1016/j.eururo.2020.11.021
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More
