Adding LuPSMA to Standard Treatment Extends OS, Delays Disease Progression in mCRPC
Adding the targeted radioligand LuPSMA to standard of care therapy for patients with metastatic castration resistant prostate cancer after androgen receptor pathway inhibition and chemotherapy extended overall survival and delayed disease progression
Michael J. Morris, MD

Adding the targeted radioligand 177Lu-PSMA-617 (LuPSMA) to standard of care therapy for patients with metastatic castration resistant prostate cancer (mCRPC) after androgen receptor (AR) pathway inhibition and chemotherapy extended overall survival (OS) and delayed disease progression, according to a presentation of the findings of the VISION trial (NCT03511664) during the Society of Nuclear Medicine and Molecular Imaging 2021 Virtual Annual Meeting.1
Eligible patients had to be treated with 1 or more AR pathway inhibitors and 1 to 2 taxane regimens. They had to have prostate-specific membrane antigen (PSMA)–positive mCRPC that was confirmed on PET/CT scan and have ECOG performance status of 0 to 2.
“The patient population for VISION had progressed through any one or more of the AR- directed therapies, and post docetaxel [Taxotere] and possibly post cabazitaxel [Jevtana],” Michael J. Morris, MD, Prostate Cancer Section head, Genitourinary Oncology, at Memorial Sloan Kettering Cancer Center in New York City, New York, said. The VISION trial had previously been presented during the 2021 American Society of Clinical Oncology Annual Meeting.2
Patients were randomized 2:1 to receive either protocol-permitted standard of care plus 177Lu-PSMA-617 at 7.4 GBq every 6 weeks for 4 cycles or standard of care alone. Alternate primary end points were radiographic progression-free survival (rPFS) and OS. “Alternate means that if either end point were significant, then the study would be positive,” Morris said. Key secondary end points included time to first symptomatic skeletal event, safety, and tolerability.
“It should be noted that 11,179 patients were screened and about 87% of these patients met the PSMA criteria, but 1000 of these patients actually underwent their PSMA scan,” Morris said. “Nearly 87% of these patients got into the trial; for 12.6% of patients, the PSMA criteria were not met.”
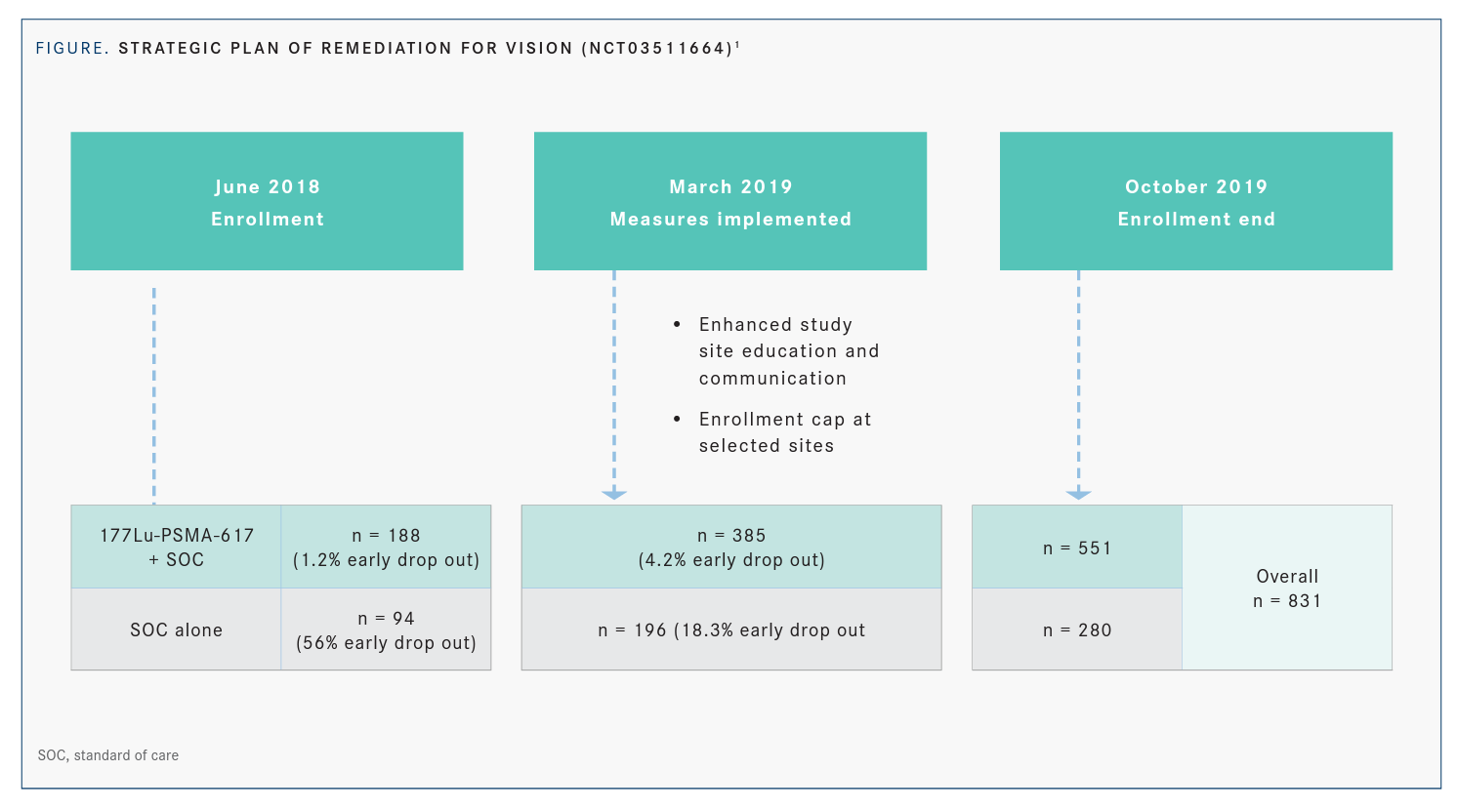
Morris explained that after about 250 patients enrolled, investigators saw a 56% dropout rate before patients started receiving standard of care. As a result, accrual was temporarily suspended and a strategic plan of remediation was implemented after discussions with the FDA (FIGURE1).
“Those measures included capping centers with a high dropout rate and re-educating investigators about the trial design and execution,” Morris said. “A special effort was focused on ensuring maximal collaboration between nuclear medicine physicians and medical oncologists.”
Once these measures were in place, the dropout rate decreased from 56% to 16%. By the time enrollment ended in October 2019, the total population was 831 patients, with 551 in the treatment arm and 280 in the control arm. In the 2 analysis sets, 581 patients were analyzed for rPFS and 831 patients were evaluated for OS.
Baseline characteristics, including race, performance status, and site of disease, were balanced across the treatment arms. Morris added that previous cancer treatments were also well balanced across treatment arms and the 2 analysis sets.
In the primary analysis of all randomized patients, investigators reported a 38% decline in risk of death for patients in the treatment arm compared with the control arm (HR, 0.62; 95% CI, 0.52-0.74; P < .01). Median survival was improved in the treatment arm vs the control arm by 4 months (15.3 months vs 11.3 months, respectively). In the rPFS analysis, the treatment arm had a 37% decline in risk of death compared with the control arm (HR, 0.63; 95% CI, 0.51-0.79). Median survival was 14.6 months for the treatment arm and 10.4 months for the control arm.
OS was generally consistent across pre-specified stratification factor subgroups, Morris said. Secondary end points such as measurable disease and PSA responses favored patients in the treatment arm.
Morris also noted postprotocol therapies and noted slightly higher rates of chemo-therapy and radiotherapy in patients in the control arm. “Postprotocol therapies are important because you want to see if there are any imbalances that might account for the [OS] interpretation in the 2 arms,” Morris said. “You ultimately want to see who went on to chemotherapy or other radiotherapies post protocols.”
Regarding safety, there was a higher rate of drug-related treatment-emergent adverse events with the addition of LuPSMA to the standard of care, Morris said. He noted that bone marrow suppression was higher in the treatment arm, although most of it was anemia or thrombocytopenia. “I don’t think this represents any new safety signals,” Morris said.
Looking ahead, Morris noted that 2 trials are looking at patient populations earlier than in the postchemotherapy setting that was involved in the VISION trial. One trial is looking at mCRPC in the prechemotherapy setting and one looks at the metastatic castration sensitive prostate cancer in the pre-CRPC setting. Overall, LuPSMA should be considered a new treatment option in patients with mCRPC, pending regulatory approval, Morris concluded.
REFERENCES
1. Morris MJ. PSMA radioligand therapy: updates from the VISION tri-al. Abstract presented at: Society of Nuclear Medicine and Molecular Imaging 2021 Virtual Annual Meeting; June 11-15, 2021; virtual. Accessed July 20, 2021. https://bit.ly/3kCJ6IW
2. Morris MJ, De Bono JS, Chi KN, et al; VISION Trial Investigators. Phase III study of lutetium-177-PSMA-617 in patients with meta-static castration-resistant prostate cancer (VISION). J Clin Oncol. 2021;39(suppl 18):LBA4. doi:10.1200/JCO.2021.39.15_suppl.LBA4

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More