Choosing Between Systemic Therapy and TACE for HCC
What is the ideal first-line<strong> </strong>therapy for nonresectable, non– transplant eligible, liver-only hepatocellular carcinoma? In a debate at the 2019 Gastrointestinal Oncology Conference, Mark Yarchoan, MD, had the unenviable task of convincing the audience that systemic therapy was the way to go.
Mark Yarchoan, MD

Mark Yarchoan, MD
What is the ideal first-linetherapy for nonresectable, non transplant eligible, liver-only hepatocellular carcinoma (HCC)? In a debate at the 2019 Gastrointestinal Oncology Conference, Mark Yarchoan, MD, had the unenviable task of convincing the audience that systemic therapy was the way to go.
“Sorafenib [Nexavar] beats supportive care, so there is clear evidence that we have systemic therapies that work,” said Yarchoan, assistant professor of oncology at Johns Hopkins Medicine in Baltimore, Maryland. “For patients with either intrahepatic or extra- hepatic [disease], there is a role for thinking about systemic therapy first.”
Many treatment guidelines, including those from the American Association for the Study of Liver Diseases, recommend transarterial chemoembolization (TACE) as first-line treatment for patients with intermediate-stage HCC.1 Yarchoan argued that systemic therapy like sorafenib improves survival, whereas TACE does not.
He cited a multicenter, double-blind, placebo- controlled phase III trial2 of 602 patients with advanced HCC who had not received prior systemic therapy, in which the median overall survival (OS) was 10.7 months in with sorafenib versus 7.9 months with placebo (HR, 0.69; 95% CI, 0.55-0.87;P<.001).
“If the patients had extrahepatic or intra- hepatic disease, it didn’t matter,” he said. “There was better survival with sorafenib than placebo. That’s been shown with virtually every study.”
Furthermore, Yarchoan added, data published in 1995 showed that lipiodol chemo- embolization did not significantly improve survival but often caused acute liver failure in patients with unresectable HCC who did not have severe liver disease.3
Michael C. Soulen, MD, director of interventional oncology at the University of Pennsylvania’s Abramson Cancer Center in Philadelphia, argued in favor of liver-directed therapy. He said that data from 6 studies show that conventional TACE improves survival more than best supportive care does.
Findings from a study conducted by the Barcelona Clinic Liver Cancer group showed that chemoembolization was superior for survival (HR, 0.47; 95% CI 0.25-0.91;P= .025), with a 2-year survival probability of 50% for embolization, 63% for chemoembolization, and 27% for control.4
However, there are more up-to-date studies showing a survival advantage for TACE, the largest of which was published inHepatologyin 2014. Results from the trial of 502 patients showed that the addition of adjuvant brivanib (BMS-582664) to TACE did not improve median OS.5
“While [the study] was negative for brivanib, it does show the effect of chemoembolization, which in both arms had a median survival of about 26 months and a 2-year survival [rate] better than 50%,” Soulen said.
Data published in October 2019 inCardioVascular and Interventional Radiology6showed a single-arm trial of 142 patients with HCC who were not eligible for curative treatment. The patients were assigned to doxorubicin-loaded HepaSphere 30 μm to 60 μm. The median OS was 31 months with an overall response rate (ORR) >80% and a 2-year OS rate >70%.
“Here we have a handful of large, prospective, controlled, modern trials all done within the past 10 years showing that the median survival for chemoembolization in these large populations is somewhere between 2 and 3 years, and 2-year survival you can expect to be better than 50%,” Soulen said.
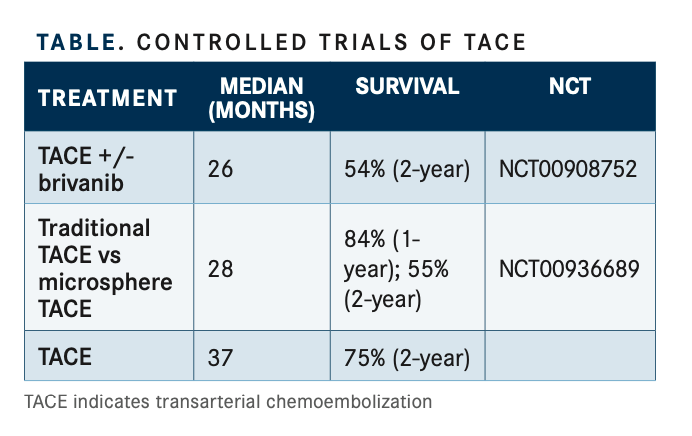
He added that real-world data support the use of TACE in this population. Findings from a systemic review of 101 articles involving 10,108 patients treated with lipiodol TACE were selected for an efficacy analysis (TABLE).7The collective ORR was 52.5% (95% CI, 43.6%-61.5%) with a 2-year OS rate of 51.8% and a 5-year OS rate of 32.4%. The median OS was 19.4 months (95% CI, 16.2-22.6).
“Liver-directed therapy is the treatment of choice for eligible patients with intermediate-to-advanced unresectable HCC,” Soulen said. He added that median OS and 2-year survival rates for TACE are double what is seen with first-line tyrosine kinase inhibitors and immune modulation agents, although that is in part because populations in drug trials are almost exclusively patients with advanced disease or those who’ve already undergone unsuccessful liver-directed therapy.
Systemic therapies, he concluded, should be reserved for those who are ineligible for TACE, those who’ve already undergone 2 unsuccessful cycles of TACE, and for those who are sequenced with liver-directed therapy for select cases of advanced disease.
References
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723- 750. doi: 10.1002/hep.29913.
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepato- cellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi: 10.1056/ NEJMoa0708857.
- Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire. A comparison of lipiodol chemoembolization and conservative treatment for unresectable hepatocellular carcinoma.N Engl J Med. 1995;332(19):1256- 1261. doi: 10.1056/NEJM199505113321903.
- Llovet JM, Real MI, Montaña X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial.Lan- cet. 2002;359(9319):1734-1739. doi: 10.1016/S0140-6736(02)08649-X.
- Kudo M, Han G, Finn RS, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial.Hepatology. 2014;60(5):1697-1707. doi: 10.1002/hep.27290.
- Malagari K, Moschouris H, Kiakidis T, et al. Five-years outcome analysis of 142 consecutive hepatocellular carcinoma patients treated with doxorubicin eluting microspheres 30-60 μm: results from a single-centre prospective phase II trial.Cardiovasc Intervent Radiol. 2019;42(11):1551- 1562. doi: 10.1007/s00270-019-02260-3.
- Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data.Hepatology. 2016;64(1):106-116. doi: 10.1002/hep.28453.

Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More