Nonbiomarker, Nonsquamous NSCLC: Diagnosis, Statistics, Staging, and Testing
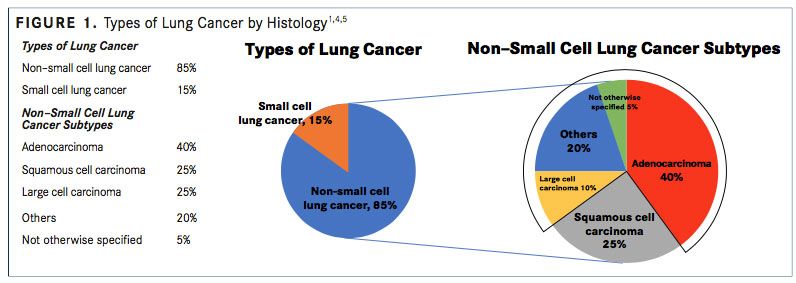
Non-small cell lung cancer is the most common type of lung cancer, accounting for approximately 80% to 85% of lung cancer cases, whereas small cell lung cancer comprises approximately 10% to 15% of lung cancer cases.
1NSCLC originates from lung epithelial cells of the central bronchi to terminal alveoli,2nonsquamous NSCLC will generally originate in peripheral lung tissue, and squamous cell carcinoma (SCC) primarily originates near a central bronchus.
Lung cancer, including both NSCLC and SCLC, contributes to the most cancer-related deaths in the United States, with 155,870 deaths estimated for 2017.2Molecularly targeted therapies have been developed based on the identification of mutations in lung cancer, a move that has led to improved survival in specific subsets of patients with metastatic disease.3Unfortunately, patients who do not harbor these mutations do not benefit from these new treatments and continue to experience poor outcomes. Being aware of current approaches to NSCLC diagnosis and assessment of disease criteria, relevant to current treatment selection strategies, is therefore a prerequisite for tailoring treatment for each patient.
Epidemiology and Risk Factors
NSCLC includes nonsmall cell carcinoma not otherwise specified (<5%), SCC (25%- 30%), and nonsquamous carcinoma (adenocarcinoma, large cell, and undifferentiated carcinoma; 70%-75%) (FIGURE 1).4,5A recent systematic review reported that the median age at diagnosis of advanced NSCLC ranged between 59 and 68 years.6,7
An estimated 80% of patients with NSCLC receive an initial diagnosis after their cancer has already spread to regional lymph nodes or has metastasized, leading to 5-year survival rates of patients with stage IV cancer of <5%.4,5,7A recent study’s results revealed that patients with nonsquamous NSCLC without known actionable mutations had a median overall survival (OS) of 10 months (95% CI, 9.4-10.8).8Also, the results of a prospective cohort study of patients with metastatic NSCLC conducted in Australia revealed that patients with actionable mutations had a significant survival advantage compared with patients without actionable mutations (HR, 0.49; 95% CI, 0.33-0.71; P <.01).9
Smoking is the greatest risk factor for developing lung cancer, contributing to an approximate 10-fold increased risk compared with lifetime nonsmokers.2The odds ratio estimate of developing lung cancer for patients who currently smoke versus patients who never smoked was 23.9 (95% CI, 19.7-29.0) in men and 8.7 (95% CI, 7.4-10.3) in women.10,11
Patients who have never smoked also develop NSCLC, with studies reporting frequencies up to approximately 30% (Japan).12,13Although it has been suggested that lung cancer in never-smokers is a separate disease, descriptive studies are lacking, with many not including a comparison group of ever-smokers.14-16A recent retrospective study reported that 22.4% of patients with newly diagnosed NSCLC were never-smokers and significantly more likely to be female (P <.001), older (P = .019), and have adenocarcinoma (P <.001).17
Other risk factors for lung cancer include secondhand smoke exposure; radiation exposure from radiation therapy to the chest or breast, radon exposure, medical imaging tests (ie, CT scans), or atomic bomb radiation; exposure to asbestos, nickel, chromium, arsenic, beryllium, soot, or tar in the workplace; chronic exposure to air pollution; a family history of lung cancer; HIV infection; and heavy smokers who take beta carotene supplements.2,18
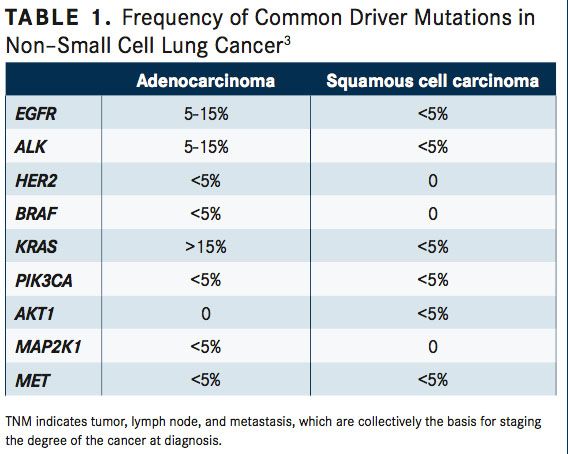
Systemic anticancer therapy recommendations for patients with stage IV NSCLC depend on individual tumor histology, patient performance status, and driver oncogene biomarker status, which are most often anaplastic lymphoma kinase (ALK) translocations and epidermal growth factor receptor (EGFR) mutations (TABLE 1).18-21Approximately 83% to 85% of patients with stage IV NSCLC do not have activating EGFR mutations or ALK translocations, requiring distinct first-line therapy recommendations.
Common sites of metastasis are other areas of the lung, the adrenal gland, and the brain.22Of patients with advanced NSCLC, 25% to 44% experience dissemination in the central nervous system, particularly in patients diagnosed with adenocarcinoma.23, 24Patients with brain metastases have particularly poor prognoses, leading to a median OS of 4 to 11 weeks or 4 to 15 months in untreated and treated patients, respectively.24,25
Evolving Methods of Nonbiomarker NSCLC Diagnosis
Lung cancer may be found incidentally on chest imaging or may present through signs and symptoms resulting from the location of the primary tumor, compression of adjacent structures, or distant metastases.2Worsening cough or chest pain are the most frequently reported symptoms at presentation; other presenting symptoms include malaise, weight loss, dyspnea, hemoptysis, and hoarseness. Dysphagia, hoarseness, and facial edema and distension of the superficial head and neck veins may be caused by local invasion or compression of the esophagus, laryngeal nerves, or the superior vena cava, respectively. Neurological defects or personality changes may also be present, resulting from brain metastases or pain from bone metastases. Upon physical examination, findings may include enlarged supraclavicular lymphadenopathy, unresolved pneumonia, pleural effusion or lobar collapse, or signs of chronic obstructive pulmonary disease or pulmonary brosis, which are associated diseases.
To make a lung cancer diagnosis, several procedures are needed, including patient history, physical examination, laboratory evaluations, chest x-ray, chest CT scan with contrast material infusion, and biopsy.2Other procedures may include:
• Sputum cytology involves obtaining sputum, or mucus coughed up from the lungs, which is viewed under a microscope by a pathologist to check for cancer cells.
• A fine-needle aspiration biopsy removes tissue or fluid from the lung using a thin needle, which is then examined under a microscope.
• The purpose of a bronchoscopy is to examine the trachea and large airways of the lung for abnormalities. The bronchoscope, a thin, tube-like instrument with a light, is inserted through the nose or mouth into the trachea and lungs.
• For a thoracoscopy, an incision is made between ribs to insert a thoracoscope, a thin, tube-like instrument with a light, to examine organs inside the chest for abnormalities. A thoracentesis is a procedure in which fluid is removed from the space between the chest lining and the lung using a needle. The fluid is then examined under a microscope by a pathologist.
• Immunohistochemistry (IHC) and electron microscopy are valuable for diagnosis and subclassification; however, most lung tumors can be classified by light microscopic criteria.
Histology and Classification
The World Health Organization/International Association for the Study of Lung Cancer have classified malignant nonsmall cell epithelial lung tumors.2,21The 3 main types of NSCLC are large cell carcinoma (10%), SCC (25%), and adenocarcinoma (40%) (FIGURE 1), with numerous less frequent subtypes. In large cell carcinoma, the cancer may originate from several types of cells that appear large and abnormal under a microscope. SCC originates in squamous cells, which are thin, at cells that look like fish scales under a microscope. These cells are found in the tissue of the skin surface, the lining of the respiratory and digestive tracts, and the lining of hollow body organs. Adenocarcinoma originates in glandular or secretory cells that line the alveoli and produce mucus and other substances.
According to the 2017 guidelines from the National Comprehensive Cancer Network (NCCN), biomarker testing is recommended for all patients presenting with stage IV NSCLC.26,27Gregory L. Riely, MD, vice chair, Clinical Trials Office, Department of Medicine, Memorial Sloan Kettering Cancer Center, and a member of the NCCN panel for NSCLC explained, “Optimally, it should be done at diagnosis; if it can’t be done then, testing before giving second-line [or any subsequent line] therapy has value.”27
Molecular testing should investigate EGFR mutations, ALK gene arrangements, ROS1 expression, and programmed death ligand-1 (PD-L1) expression.26-28These biomarkers can be DNA- or protein-based, requiring detection through sequencing and fluorescence in situ hybridization (FISH) of IHC, respectively. Sequencing is used to detect EGFR,BRAF, and MET exon 14 mutations. FISH is used to detect ROS, RET, and ALK. IHC is used to detect ALK, ROS1, PD-L1, and occasionally, EGFR mutations. Other driver oncogenes can be altered in NSCLC, including KRAS, HER2, RET fusions,BRAFV600E, and MET exon 14 alterations.27Furthermore, other genotypes contribute to 50% of NSCLC diagnoses. Yet, EGFR and ALK mutations are most common in nonsmoking patients with adenocarcinomas, whereas KRAS and BRAF mutations are more commonly found in current or former smokers.2
EGFR-mutant NSCLC can be treated more effectively upfront with erlotinib (Tarceva), gefitinib (Iressa), or afatinib (Gilotrif) than platinum doublets. No current therapies target KRAS. ALK fusions can currently be treated with ceritinib (Zykadia), alectinib (Alecensa), and crizotinib (Xalkori);BRAFV600E can be treated with the combination of trametinib (Mekinist) and dabrafenib (Tafinlar); ROS1 fusions and MET exon 14 can be treated with crizotinib; and RET fusions can be treated with cabozantinib (Cabometyx).
Several factors associated with adverse prognoses have been identified, including the presence of pulmonary symptoms, nonsquamous histology, tumor size larger than 3 cm, metastases to multiple lymph nodes, and vascular invasion.28-30
Nonbiomarker NSCLC Staging
Disease stage plays a critical role in the selection of therapy based on both clinical and pathological factors.31To determine
staging, procedures used to diagnose NSCLC may be used, with the addition of uorodeoxyglucose-positron emission tomography (FDG-PET) scanning. Tissue samples may be obtained via bronchoscopy, mediastinoscopy, or anterior mediastinotomy:
• A mediastinoscopy is a procedure in which an incision is made at the top of the breastbone and a mediastinoscope is inserted into the chest to examine the organs, tissues, and lymph nodes between the lungs for abnormalities.
• An anterior mediastinotomy also uses a mediastinoscope, but is inserted next to the breastbone to examine the organs and tissues between the lungs and between the breastbone and heart. Pathological staging of NSCLC involves examination of the tumor, margin resection, and lymph nodes.
At diagnosis, patients with NSCLC can be classified into 3 groups that reflect the disease extent and the treatment approach, including surgically resectable disease, locally and/or regionally advanced disease, and distant metastatic disease.32Patients with surgically resectable disease are generally stages I, II, and selected stage III tumors. These patients have the best prognosis, depending on other tumor and host factors. Curative radiation therapy may be used in patients who have medical contraindications to surgery. Locally (T3-T4) and regionally (N2- N3) advanced diseases have a diverse natural history, as these patients may benefit from combination treatments. Patients with unresectable disease are often treated with combined radiation and chemotherapy, whereas selected patients can be effectively treated with surgical resection and chemoradiation therapy or preoperative/postoperative chemotherapy. Patients with distant metastatic disease may be treated with chemotherapy or radiation therapy for palliation of symptoms from the primary tumor.
If accurate evaluation of the nodal status is necessary for therapy determination, surgical staging of the mediastinum is considered standard.32CT scanning, primarily used to determine tumor size, should extend inferiorly to include the liver and adrenal glands. No advantage has been reported for MRI scans of the thorax and upper abdomen.33FDG-PET scanning may facilitate identification of patients with metastatic disease that is beyond the scope of surgical resection and is missed by standard preoperative staging procedures, leading to improved patient outcomes.34Using CT imaging and FDG-PET scanning synergistically has improved specificity and sensitivity compared with CT imaging alone. CT or MRI scans may be used to stage patients at risk for brain metastases.32For patients unable to tolerate MRI, contrast-enhanced CT scan may be used.
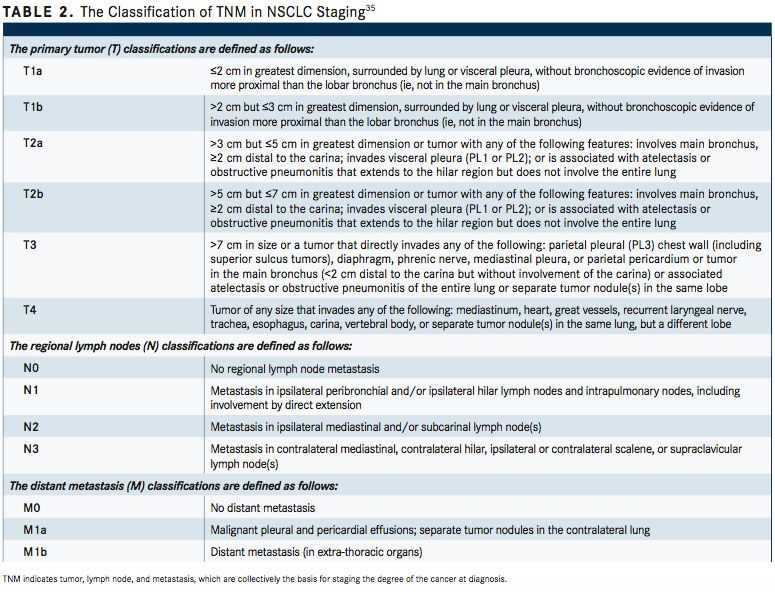
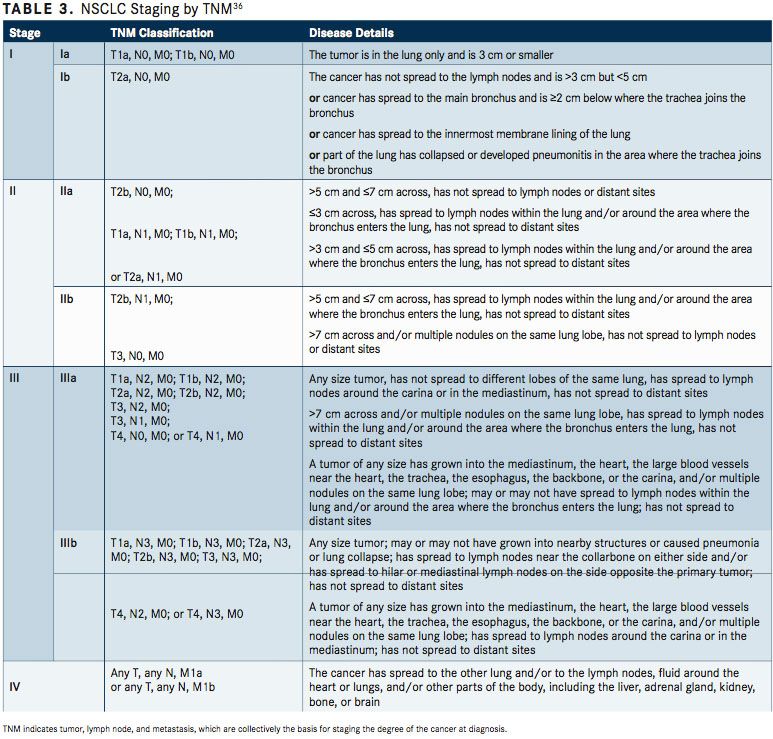
The Revised International Staging System for Staging Lung Cancer was adopted in 2010 by the Union Internationale Contre le Cancer and the American Joint Committee on Cancer (AJCC) (TABLE 2).35To define NSCLC, the AJCC has designated staging by TNM classification.36The occult carcinoma stage is defined as TX, N0, M0 disease. TX represents primary tumors that cannot be assessed or tumors that have been proven based on the presence of malignant cells in sputum or bronchial washings that have not been visualized by bronchoscopy or imaging. TNM stage 0 is defined as Tis, N0, M0, with Tis representing carcinoma in situ (TABLE 3). Overall, appropriate and timely NSCLC diagnosis is crucial for treatment planning and prognosis determination. In an issue of American Family Physician, Kelly M. Latimer, MD, MPH, and Timothy F. Mott, MD, both of the Naval Hospital, Pensacola, Florida, explain, “The diagnostic evaluation and treatment of a patient with lung cancer require a team of specialists, including a pulmonologist, medical oncologist, radiation oncologist, pathologist, radiologist, and a thoracic surgeon. Cancer specimens are tested for various mutations, which, if present, can be treated with new targeted molecular therapies.”37
Unfortunately, patients with nonsquamous NSCLC who do not have actionable mutations will not benefit from these new targeted therapies and often have a poorer prognosis. Further studies are needed to improve outcomes for this patient population.
References:
- American Cancer Society. What is non-small cell lung cancer? ACS website. cancer. org/cancer/non-small-cell-lung-cancer/about/what-is-non-small-cell-lung-cancer. html. Updated May 16, 2016. Accessed August 2, 2017.
- National Cancer Institute. Non-small cell lung cancer treatment (PDQ)-Health Professional Version. NCI website. cancer.gov/types/lung/hp/non-small-cell-lung-treat- ment-pdq. Updated March 31, 2017. Accessed August 2, 2017.
- Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12(2):175-180. doi: 10.1016/S1470-2045(10)70087-5.
- Howlader N, Noone AM, Krapcho M, et al (eds). Surveillance Epidemiology and End Results (SEER) Program: SEER cancer statistics review (CSR) 1975-2012. National Cancer Institute website. seer.cancer.gov/csr/1975_2012/. Updated November 18, 2015. Accessed August 3, 2017.
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359(13):1367- 1380. doi: 10.1056/NEJMra0802714.
- Davies J, Patel M, Gridelli C, de Marinis F, Waterkamp D, McCusker ME. Real-world treatment patterns for patients receiving second-line and third-line treatment for ad- vanced non-small cell lung cancer: a systematic review of recently published studies. PLoS ONE. 2017;12(4):e0175679. doi: 10.1371/journal.pone.0175679.
- SEER cancer statistics factsheets: lung and bronchus cancer, 2015. National Cancer Institute website. seer.cancer.gov/statfacts/html/lungb.html. Accessed August 3, 2017.
- Abernethy AP, Arunachalam A, Burke T, et al. Real-world rst-line treatment and over- all survival in non-small cell lung cancer without known EGFR mutations or ALK rear- rangements in US community oncology setting. PLoS ONE. 2017;12(6):e0178420. doi: 10.1371/journal.pone.0178420.
- Tan L, Alexander M, Of cer A, et al. Survival difference according to mutation status in a prospective cohort study of Australian patients with metastatic non-small cell lung carcinoma (NSCLC). Intern Med J. 2017. doi: 10.1111/imj.13491.
- Simonato L, Agudo A, Ahrens W, et al. Lung cancer and cigarette smoking in Europe: an update of risk estimates and an assessment of inter-country heterogeneity. Int J Cancer. 2001;91:876
- Agudo A, Ahrens W, Benhamou E, et al. Lung cancer and cigarette smoking in women: a multicenter case-control study in Europe. Int J Cancer. 2000;88(5):820-827. doi: 10.1002/1097-0215(20001201)88:5<820::AID-IJC21>3.0.CO;2-J.
- Asahina H, Sekine I, Horinouchi H, et al. Retrospective analysis of third-line and fourth-line chemotherapy for advanced non-small-cell lung cancer. Clin Lung Cancer. 2012;13(1):39-43. doi: 10.1016/j.cllc.2011.06.010.
- Zhou W, Christiani DC. East meets West: ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and Caucasians. Chin J Cancer. 2011;30(5):287-292. doi: 10.5732/cjc.011.10106.
- Couraud S, Souquet PJ, Paris C, et al; French Cooperative Intergroup IFCT. BioCAST/ IFCT-1002: epidemiological and molecular features of lung cancer in neversmokers. Eur Respir J. 2015;45(5):1403-1414. doi: 10.1183/09031936.00097214.
- Toh CK, Lim WT. Lung cancer in never-smokers. J Clin Pathol. 2007;60(4):337-340. doi: 10.1183/09031936.00046915.
- Rudin CM, Avila-Tang E, Harris CC, et al. Lung cancer in never smokers: molecular pro les and therapeutic implications. Clin Cancer Res. 2009;15(18):5646-5661. doi: 10.1158/1078-0432.CCR-09-0377.
- Dias M, Linhas R, Campainha S, Conde S, Barroso A. Lung cancer in never- smokers what are the differences? Acta Oncologica. 2017;56(7):931-935. doi: 10.1080/0284186X.2017.1287944.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guide- lines in Oncology (NCCN Guidelines): non-small cell lung cancer. Version 8.2015. NCCN website. nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed Au- gust 7, 2017.
- Masters GA, Temin S, Azzoli CG, et al; American Society of Clinical Oncology Clini- cal Practice. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2015;33(30):3488-3515. doi: 10.1200/JCO.2015.62.1342.
- Reck M, Popat S, Reinmuth N, et al; ESMO Guidelines Working Group. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagno- sis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii27-39. doi: 10.1093/ annonc/mdu199.
- Travis WD, Brambilla E, Nicholson AG, et al; WHO Panel. The 2015 World Health Organization classi cation of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classi cation. J Thorac Oncol. 2015;10(9):1243-1260. doi: 10.1097/JTO.0000000000000630.
- National Comprehensive Cancer Network (NCCN). NCCN Guidelines for Patients: Lung Cancer. Version 1.2016. NCCN website. nccn.org/patients/guidelines/lung- nsclc/ les/assets/basic-html/page-30.html. Accessed August 7, 2017.
- Langer CJ, Mehta MP. Current management of brain metastases, with a fo- cus on systemic options. J Clin Oncol. 2005;23(25):6207-6219. doi: 10.1200/ JCO.2005.03.145.
- Besse B, Le Moulec, Sénéllard H, et al. A phase II trial of bevazicumab in combination with first-line chemotherapy or second-line erlotinib in nonsquamous NSCLC patients with asymptomatic untreated brain metastases. Poster presented at: 2012 ESMO Annual Congress; September 28-October 2, 2012; Vienna, Austria. Abstract 1299P.
- Sundstrom JT, Minn H, Lertola KK, Nordman E. Prognosis of patients treated for intracranial metastases with whole-brain irradiation. Ann Med. 1998;30(3):296-299.
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guide- lines in Oncology (NCCN Guidelines): non-small cell lung cancer. Version 8.2017. NCCN website. nccn.org/professionals/physician_gls/pdf/nscl_blocks.pdf. Accessed August 10, 2017.
- Riely GL. What, when, and how of biomarker testing in non-small cell lung cancer. J Natl Compr Canc Netw. 2017;15(5S):686-688. doi: 10.6004/jnccn.2017.0073.
- Ichinose Y, Yano T, Asoh H, Yokoyama H, Yoshino I, Katsuda Y. Prognostic factors ob- tained by a pathologic examination in completely resected non-small-cell lung cancer. An analysis in each pathologic stage. J Thorac Cardiovasc Surg. 1995;110(3):601-605.
- Osaki T, Nagashima A, Yoshimatsu T, Tashima Y, Yasumoto K. Survival and charac- teristics of lymph node involvement in patients with N1 non-small cell lung cancer. Lung Cancer. 2004;43(2):151-157. doi: 10.1016/j.lungcan.2003.08.020.
- Khan OA, Fitzgerald JJ, Field ML, et al. Histological determinants of survival in completely resected T1-2N1M0 nonsmall cell cancer of the lung. Ann Thorac Surg. 2004;77(4):1173-1178.
- P ster DG, Johnson DH, Azzoli CG, et al; American Society of Clinical Oncology. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol. 2004;22(2):330-353. doi: 10.1200/ JCO.2004.09.053.
- National Cancer Institute. Non-small cell lung cancer treatment (PDQ)-Health Pro- fessional Version. Stage information for NSCLC. cancer.gov/types/lung/hp/non- small-cell-lung-treatment-pdq#link/_478_toc. Updated March 31, 2017. Accessed August 2, 2017.
- Webb WR, Gatsonis C, Zerhouni EA, et al. CT and MR imaging in staging non-small cell bronchogenic carcinoma: report of the Radiologic Diagnostic Oncology Group. Radiology. 1991;178(3):705-713. doi: 10.1148/radiology.178.3.1847239.
- Vansteenkiste JF, Stroobants SG, De Leyn PR, et al. Lymph node staging in non- small-cell lung cancer with FDG-PET scan: a prospective study on 690 lymph node stations from 68 patients. J Clin Oncol. 1998;16(6):2142-2149. doi: 10.1200/ JCO.1998.16.6.2142.
- Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111(6):1710-1717.
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010:253-270.
- Latimer KM, Mott TF. Lung cancer: diagnosis, treatment principles, and screening. Am Fam Physician. 2015;91(4):250-256.
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More