Randall Reviews Efficacy and Safety of Niraparib as Primary Maintenance for Ovarian Cancer
During a Targeted Oncology™ Case-Based Roundtable™ event, Leslie M. Randall, MD, MAS, evaluated the outcomes of the PRIMA/ENGOT-OV26/GOG-3012 study of niraparib as primary maintenance therapy for patients with ovarian cancer.
Leslie M. Randall, MD, MAS
Professor and Director
of Gynecologic Oncology
Virginia Commonwealth University
Richmond, VA

CASE SUMMARY
- A 49-year-old woman presented to her primary care physician complaining of abdominal bloating and nausea.
- Medical history: mild hypertension
- Family history: Mother died of breast cancer at age 59; cousin on mother’s side died of ovarian cancer at age 65.
- CT: small-volume ascites, bilateral 8-cm adnexal masses
- Cancer antigen 125, 285 U/mL
- She underwent exploratory laparotomy followed by omentectomy, bilateral salpingo-oophorectomy, and resection of peritoneal nodules for stage IIIC high-grade serous carcinoma.
- Optimal cytoreduction with < 1 cm of residual disease after surgery
Targeted Oncology: What data support the use of niraparib (Zejula) as first-line maintenance therapy after platinum-based chemotherapy?
RANDALL: The SOLO-1 study [NCT01844986] was amazing. We were all very excited to see the results, but they were restricted to our population with BRCA [mutations].1 We didn’t have an option for PARP inhibitor maintenance in the frontline setting outside that population.
The PRIMA/ENGOT-OV26/GOG-3012 study [NCT02655016] was the first to expand access to PARP inhibitors in patients who [either] had or did not have BRCA mutations. They enrolled patients with newly diagnosed advanced ovarian cancer at high risk for recurrence after response to first-line platinum-based chemotherapy. That meant…they either had neoadjuvant chemotherapy and had a complete or partial response or had residual disease after frontline surgery. Randomization was 2:1 to niraparib vs placebo.
The primary end point was progression-free survival [PFS], but it was by a blinded independent review instead of the investigator’s assessment. The key secondary end point was overall survival [OS], and then the times to first and subsequent therapies were also secondary end points.
The stratification factors were whether they had neoadjuvant chemotherapy; their best response to frontline platinum therapy, either complete or partial response; and their tissue homologous recombination status, either homologous recombination deficient [HRd] or homologous recombination proficient [HRp] tumors. An individualized starting dose and hierarchical PFS testing were used. They tested HRd patients first. If the hazard ratio was positive in that group, then they would have statistical power to test the overall population.2
The baseline demographics were very similar to [those of] the SOLO-1 study. The average age was 62 [years, and] most patients had stage IIIC or IV disease. Approximately 67% of patients had prior neoadjuvant chemotherapy, and that’s a little different from SOLO-1. It reflects the incorporation of more neoadjuvant therapy into our treatment protocols over time because this trial happened after SOLO-1. Most patients had at least 6 cycles of chemotherapy, some had a little bit more—approximately 25%—and most had high-grade serous tumors.2
How did longer-term follow-up affect PFS?
Patients had an [initial] PFS benefit with the hazard ratio approaching what we saw in SOLO-1 but not quite as dramatic [overall population hazard ratio, 0.62; 95% CI, 0.50-0.76; P < .001]. When we break this down by biomarker subgroup, the largest magnitude of benefit consistent with SOLO-1 was in the HRd BRCA-mutated group [HR, 0.43; 95% CI, 0.31-0.59; P < .0001].
The next best would be the HRd BRCA wild-type, and then the HRp patients having some benefit but not quite as marked as in the other biomarker subgroups. These data supported the approval of niraparib for all patients with ovarian cancer in the frontline setting. Some physicians stopped testing the biomarkers when the PRIMA data became available because they said, “I’ll just put them on niraparib, and I don’t need to test.” That’s true. The only test that I advocate for patients is germline BRCA testing for their own breast cancer risk assessment and for the benefit of their blood relatives.
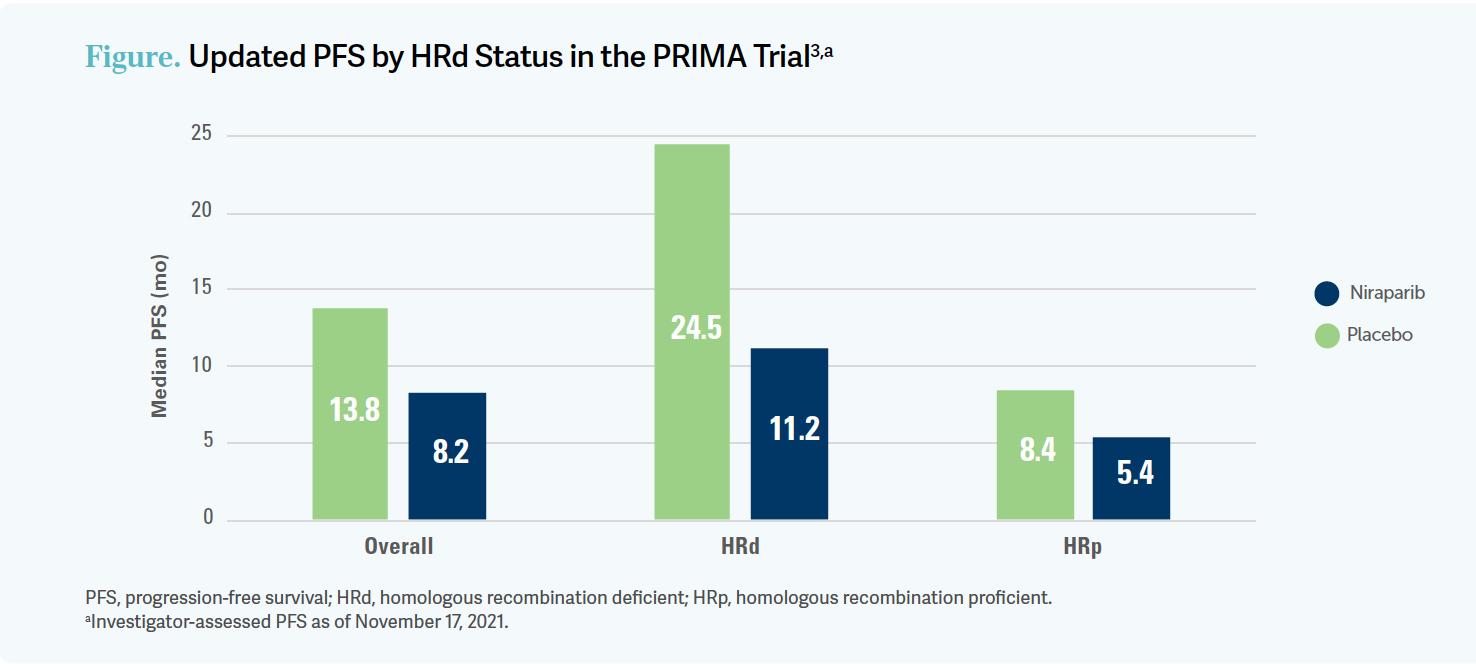
The updated long-term PFS data showed an advantage in both the HRd group with a hazard ratio of 0.45 [95% CI, 0.32-0.64], which was highly significant, and the HRp [group] showing a hazard ratio of 0.65 [95% CI, 0.48-0.87], which was also statistically significant, but again a smaller magnitude of benefit.3
Cross-trial comparisons are not statistically fair, but we all use them to inform our practice. These data are about the same as for bevacizumab [Avastin] alone in this group or any ovarian cancer.
How much benefit do you need to see to recommend a therapy?
I’ll…say in my practice, all patients with HRd or BRCA-mutated tumors get PARP inhibition in some form, and in my HRp patients who need some maintenance, I use bevacizumab or a PARP inhibitor. I tend to use a PARP inhibitor because I want them to get it early so that they have a lesser risk of MDS [myelodysplastic syndrome] or AML [acute myeloid leukemia]. Also, it tends to help convert a patient who might become platinum resistant into one who is more platinum sensitive. Now, some argue that it is a biological thing, and you could artificially make them platinum sensitive by prolonging that first progression.
I don’t know what the right answer is, but I do know that I tend to use the PARP inhibitor earlier in my practice. Just like [in] SOLO-1, all patient subgroups [in the PRIMA trial] had a significant benefit from the PARP inhibitor. Probably the biggest gradation in magnitude of benefit is, again, by biomarker [Figure3]. BRCA mutated is better than HRd BRCA wild-type, which is better than HRp. Still, all 3 groups had benefit.
What is known about OS in patients receiving niraparib?
The OS data for PRIMA are not as robust as for SOLO-1…they are not as mature and as complete. We have 84% of patients on niraparib alive at 2 years vs 77% for placebo. [The data] numerically favor niraparib, but [they’re] not yet powered to determine the actual difference because [they’re] still immature, and the OS events are low, which is very interesting in this higher-risk population. Deaths on the study are still low, so the low maturity of these data is evident.
The hazard ratios by biomarker showed no difference in OS, and it crossed 1 in the HRd and overall populations. Interestingly, there’s a trend toward improved OS in the HRp population [81% vs 59% OS rate at 2 years for niraparib vs placebo, respectively; hazard ratio, 0.51; 95% CI, 0.27-0.97], which initially didn’t have the biggest magnitude of benefit.4
Still, these data are just so immature, so it’s difficult to draw solid conclusions. It seems a little bit inconsistent with the PFS data, but I would not get caught up with that. The data are just not mature.
What was the safety profile of niraparib observed in this trial?
For the safety data, patients on niraparib experienced more adverse events [AEs] than patients on placebo. Interestingly, [some] high-grade AEs occurred even in patients on placebo. Before we had placebo-controlled trials, we thought all this toxicity was due to the PARP inhibitors. But when we did placebo-controlled trials, we realized that our patients with ovarian cancer are in bad shape after all that treatment, regardless.
The PRIMA study had excess thrombocytopenia, and this was even with the individualized starting dose. There’s no question that thrombocytopenia is a little bit more difficult to manage with niraparib vs olaparib [Lynparza], as seen in SOLO-1. Also, anemia and neutropenia were an issue.
The GI [gastrointestinal] toxicity was a little bit less in the niraparib group, which mostly had hematologic toxicity. Interestingly, niraparib also has hypertension as an off-target effect. It’s not common, and when it does happen, it typically happens very quickly. That’s not common with other PARP inhibitors, and you need to watch out for it.3
Patients with the individualized starting dose had a statistically significant improvement in PFS. That benefit was still maintained even if the patient started at the lower dose, and that was true across the HRd, germline BRCA-mutated, and non– BRCA-mutated groups. Again, the OS data for the individualized dose are still immature, but the safety improved with the individualized starting dose. Thrombocytopenia, anemia, and neutropenia were much more tolerable in the individualized dosing group.5
REFERENCES
1. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495- 2505. doi:10.1056/NEJMoa1810858
2. González-Martín A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391- 2402. doi:10.1056/NEJMoa1910962
3. González-Martin A, Pothuri B, Vergote I, et al. PRIMA/ENGOT-OV26/GOG-3012 study: updated long-term PFS and safety. Ann Oncol. 2022;33(suppl 7):S235-S282. doi:10.1016/j.annonc.2022.07.658
4. Monk BJ, Han S, Pothuri B, et al. Efficacy of niraparib therapy in patients with newly diagnosed advanced ovarian cancer by BRCA and homologous recombination status: PRIMA/ENGOT-OV26/GOG-3012 study. Presented at: 2020 Society of Gynecologic Oncology Annual Meeting. April 29, 2020; Virtual. Accessed August 16, 2023. https://tinyurl. com/33c95emv
5. Zejula. Prescribing information. GSK; updated April 2023. Accessed July 12, 2023. https://tinyurl. com/2p8rztfh

Advances in Subsequent Therapies Shake Up Sequencing of ccRCC Treatment
April 25th 2024With the approval of belzutifan and other newer data for treating patients with recurrent renal cell carcinoma, the state of subsequent therapies is advancing beyond the reuse of frontline options with impacts on duration of response and quality of life.
Read More
Novel Approaches Focus on Limiting Toxicity in Older Patients With ALL
April 22nd 2024The major challenges for clinicians treating older patients with acute lymphoblastic leukemia surround the emergence of resistance to existing therapies and the toxicities associated with current chemotherapies.
Read More
Ornstein Advises on Starting Dose and Management of Lenvatinib in RCC
April 21st 2024During a Case-Based Roundtable® event, Moshe Ornstein, MD, MA, provided guidance on dosing and toxicity concerns in a patient treated with lenvatinib plus pembrolizumab for advanced renal cell carcinoma.
Read More