PARP Inhibition Changes Individualized Treatment in Advanced Ovarian Cancer
The addition of PARP inhibitors for the treatment of patients with BRCA-mutated advanced ovarian cancer has provided clinicians with a stronger set of treatment options. Looking at how the BRCA mutation responds to these inhibitors is then vital to deciding the patient’s treatment path.
Image credit: © Lars Neumann via Adobe Stock

THE STRENGTH OF targeted therapies is the ability to craft a treatment regimen that can attack cancers based on the patients’ unique biomarkers. In the case of treating patients with ovarian cancer, understanding how to provide treatment for these patients with a BRCA mutation has proven to be indispensable.
In 2023 alone, approximately 19,710 women will be given an ovarian cancer diagnosis and approximately 13,270 of these women will die from the disease, according to key statistics from the American Cancer Society.1 In the general population, this amounts to about 1.2% of women who will develop ovarian cancer in their lifetime.2 However, for women who inherit a BRCA1 variant, 39% to 44% will develop ovarian cancer by 70 to 80 years of age; 11% to 17% of women with a BRCA2 variant will experience the same outcome.3
Patients with ovarian cancer who harbor these variants inherited them from either parent, as BRCA is a familial mutation. Often, patients with a family history of ovarian or breast cancer will harbor a BRCA variant, but for patients with a BRCA variant who then develop ovarian cancer, their tumors will mutate. This makes them more resistant to traditional ovarian cancer treatment3 and will require maintenance treatment over time. Yet, the field of targeted oncology has produced PARP inhibitors, which help further cell death in tumors with an abnormal BRCA gene.
PARP enzymes and BRCA genes help repair DNA in different ways, but when BRCA genes are mutated, it becomes harder for the cell to repair DNA, and the addition of a PARP inhibitor makes the task of a tumor cell to repair that much harder.4 Moreover, patients with a BRCA1 variant could have a wild-type copy of the gene (BRCAwt), which is a risk modifier for patients with ovarian cancer and has shown to have different outcomes when treated with PARP inihibitors.5
BRCA variants are not the only cause of ovarian cancer, as 10% to 15% of patients with epithelial ovarian cancer have DNA mismatch repair deficiency (dMMR).6 dMMR occurs when there are errors in the base pairing of the DNA, which then leads to microsatellite instability (MSI) indicated by uncorrected mistakes in repetitive DNA sequences. Tumors that exhibit dMMR will demonstrate MSI and often require a different approach to their treatment.
To provide the best care possible, clinicians have to identify the biomarker makeup of their patients and how their tumor has mutated. The status of each patient’s tumor will drastically change the potential treatment options for clinicians and the treatment path clinicians will help guide their patients down.
TESTING FOR BRCA MUTATIONS TO INITIATE PARP INHIBITOR TREATMENT
According to the American Society of Clinical Oncology, their germline and somatic testing guidelines recommend that all patients with epithelial ovarian cancer, even if they don’t have a germline pathogenic or likely pathogenic BRCA1/2 variant, should undergo genetic testing.7 Further, women diagnosed with either clear cell, endometrioid, or mucinous ovarian cancer should undergo somatic testing to determine whether they have dMMR. Patients with epithelial ovarian cancer should also undergo this testing for dMMR.8
Importantly, BRCA1 and BRCA2 genes are homologous recombination genes that are supposed to aid in DNA repair. Yet once they are mutated in the patient’s tumor, their ability to repair DNA is damaged, allowing for further tumor growth.9 Thus, identifying in these patients whether they remain homologous recombination deficient (HRd) or homologous recombination proficient (HRp) is another factor that will change the direction of care.
PARP inhibitors that can be considered for these patients include olaparib (Lynparza), rucaparib (Rubraca), and niraparib (Zejula), which are all FDA approved for patients with ovarian cancer with a BRCA mutation.10-12 However, these are all used as first-line maintenance therapy, as patients will first undergo either primary debulking surgery if they are fit, or neoadjuvant chemotherapy until they are fit for surgery or ready for the carboplatin plus paclitaxel chemotherapy regimen.13 In addition to the platinum-based chemotherapy, they will be given either bevacizumab (Avastin) or a PARP inhibitor, depending on their germline and somatic tumor testing.
“With the tumor testing, there are some clinical decisions that can be informed [with it]; however, it’s not necessary if you’re [thinking] PARP therapy for all,” explained Ritu Salani, MD, MBA, who led a Case-Based Roundtable event, in California, on the use of PARP inhibition for patients with ovarian cancer. “If you’re going to withhold maintenance, or you don’t believe in maintenance therapy for HRp patients or HRd-negative [patients], then I think tumor testing is important. You don’t want to miss those patients who have a somatic BRCA mutation or are HRd positive, because there are significant differences in those patient populations.”
Further, discussing the initiation of PARP inhibitor treatment with a patient is important to help them understand the road ahead. Chad Hamilton, MD, leading another Case-Based Roundtable event on treating patients with BRCA-positive advanced ovarian cancer, told the physicians in attendance from Louisiana and Texas that he explains to his patients how the risk for secondary malignancies with PARP inhibitors is much lower than the risk for disease recurrence if they do not take a PARP inhibitor.14 However, this does not mean that the risk of secondary malignancies such as myelodysplastic syndrome [MDS] should be overlooked in this patient population, according to Hamilton.
“I’ve had a couple of patients who had profound drops in their blood counts, and it scares the heck out of me, but they were persistent and recovered. But I always wonder when that happens, did I talk to them enough about that risk [for MDS],” Hamilton said.
BRCA TREATMENT CONSIDERATIONS
According to Salani, a gynecologic oncologist and the University of California, Los Angeles, gynecologic oncology fellowship director, one of the considerations for patients with a BRCA mutation undergoing treatment with a PARP inhibitor is that they may switch the inhibitor they are being treated with. This can happen depending on their response to treatment but also in the gap of time between other steps of treatment, or if they display potential resistance to platinum chemotherapy. However, this is an approach Salani does not endorse because she says there is not much benefit, if any at all, to be found for patients changing their PARP inhibition treatment.
For a patient with a BRCAwt gene that is HRd positive, niraparib would be initiated only if the patient did not receive bevacizumab along with their platinum chemotherapy. Conversely, results from the randomized, double-blind, international phase 3 PAOLA-1 study (NCT02477644) initially showed a significant progression-free survival (PFS) benefit for these patients when given olaparib in addition to bevacizumab.15
This survival benefit was confirmed in a 5-year follow-up overall survival (OS) analysis of patients with a BRCA mutation who were HRd positive or negative. Higher-risk patients with a BRCA1/2 mutation in their disease on the combination of olaparib and bevacizumab (n = 109) had a 65.2% OS rate compared with 48.3% for patients on placebo and bevacizumab (n = 55; HR, 0.69; 95% CI, 0.43-1.12). Lower-risk patients in the mutated group on the combination therapy (n = 48) compared with placebo (n = 25) had a 91.4% 5-year OS rate vs 66.1%, respectively (HR, 0.27; 95% CI, 0.08-0.80).15
Patients with high-risk disease who were also HRd positive (n = 177) saw a similar benefit on the PARP inhibitor, with a 5-year OS rate of 55.2% compared with patients on placebo and bevacizumab (n = 89) at 42.2% (HR, 0.70; 95% CI, 0.50-1.00). HRd-positive patients with low-risk disease on the combination therapy (n = 78) had an OS rate of 88.3% compared with 61.3% for those in the placebo arm (n = 43; HR, 0.31; 95% CI, 0.14-0.66).
Still, every patient is an individual, and how their disease presents requires a tailored approach. In both of the Case-Based Roundtable events on first-line maintenance therapy for patients with ovarian cancer, a hypothetical case was used of a 49-year-old woman with BRCAwt and HRd-positive ovarian cancer who had a cancer antigen-125 level of 285 U/mL before surgery. The patient then underwent exploratory laparotomy followed by omentectomy, bilateral salpingo-oophorectomy, and resection of peritoneal nodules for stage IIIC high-grade serous carcinoma. Optimal cytoreduction showed less than 1 cm of residual disease after surgery.
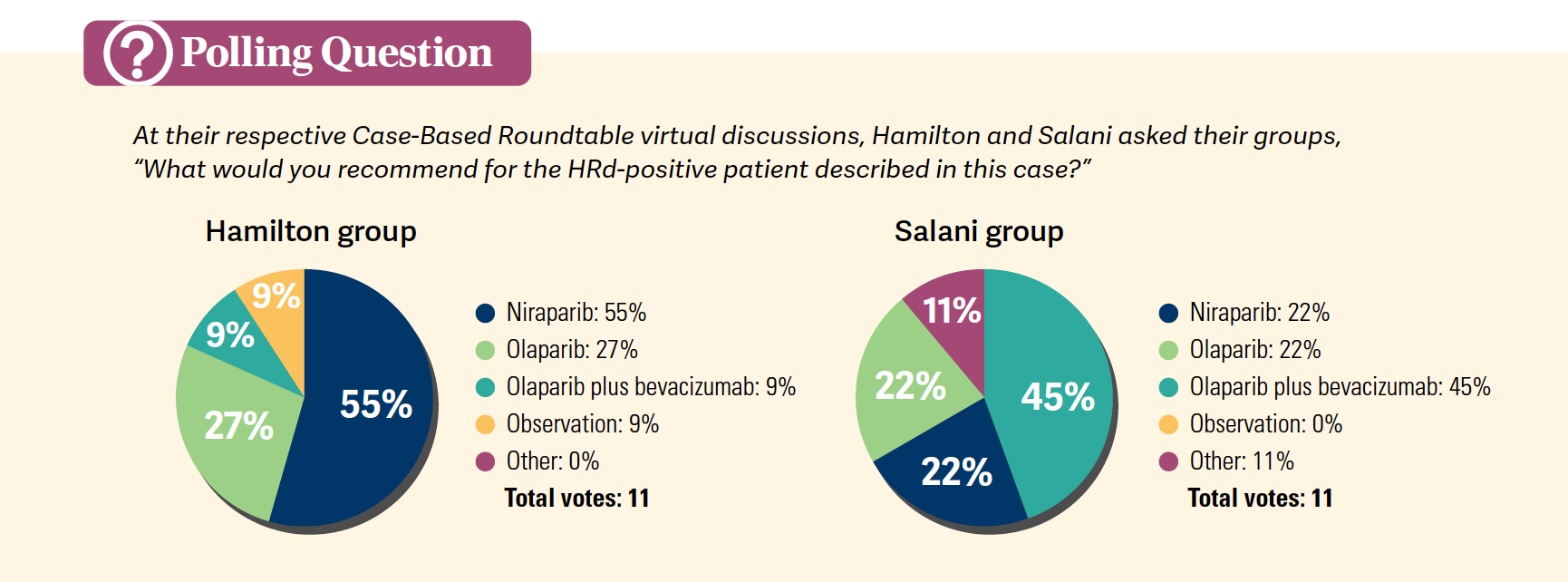
According to Hamilton, a surgical oncologist and associate research director at the Ochsner Cancer Institute in New Orleans, Louisiana, this was a patient for whom he probably wouldn’t have started bevacizumab in the front line because he believes the patient’s disease volume was low and they had a good response to surgery. Therefore, he would not have started bevacizumab but given niraparib monotherapy instead, based on the weights and platelets dosing chart, as maintenance treatment. However, this approach is not one-size-fits-all; 9% of physicians in his live event still wanted to give the patient in this case olaparib plus bevacizumab when asked in a poll, but 55% agreed with Hamilton’s niraparib monotherapy approach [Polls].
“In the community, there is a tendency to add [bevacizumab] for practically every patient with advanced ovarian cancer. But most of the benefit seems to have accrued in those who had pleural effusions,” explained Dilip Solanki, MD, a medical oncology/hematology physician with Texas Oncology, at Hamilton’s event.
FIRST-LINE PARP MONOTHERAPY
Data for both physicians’ choice of using niraparib monotherapy came from the phase 3 PRIMA/ENGOT-OV26/GOG-3012 trial (NCT02655016) that randomly assigned 733 patients with newly diagnosed advanced ovarian cancer—373 of those patients being HRd positive with BRCA mutations—to niraparib or placebo.16 Patients on niraparib were given a fixed dose of the PARP inhibitor at 300 mg (n = 481), but an individualized dose of either 200 mg or 300 mg was given if patients had a body weight less than 77 kg and/or a platelet count of less than 150,000 cells/uL vs a body weight and/or platelet count greater than these (n = 247), respectively.16
At the 2022 European Society for Medical Oncology Gynaecological Cancers Congress, an update of the PRIMA trial showed a durable PFS rate for patients on niraparib at a median follow-up of 3.5 years.17 The median PFS for patients in the HRd population on niraparib was significantly better vs placebo at 24.5 months vs 11.2 months, respectively (HR, 0.52; 95% CI, 0.40-0.68; P < .001). The overall population maintained this benefit at a median PFS of 13.8 months for patients on niraparib vs 8.2 months on the placebo arm (HR, 0.66; 95% CI, 0.56-0.79; P < .001). Further, among subgroups of patients with a BRCA mutation, those patients with HRd-positive tumors that were also BRCA mutated experienced a better PFS result with the PARP inhibitor as well (HR, 0.45; 95% CI, 0.32-0.64). In patients with HRp tumors, the PARP inhibitor still displayed a better PFS benefit with an HR of 0.65 (95% CI, 0.49-0.87), while only 9.2% of patients who received niraparib had to receive a subsequent PARP inhibitor therapy.17
According to Salani, this showed that if patients on niraparib hadn’t shown disease recurrence after 3 years from treatment, then there is a low likelihood that they will have disease recurrence. Moreover, she highlighted that patients with HRp tumors given placebo in this study had a median PFS of 6 months vs about 8 months on niraparib. While this is not as pronounced of a difference as in patients with HRd and/or BRCA-mutated tumors, for her, it is still enough to give the PARP inhibitor the benefit of the doubt. She explained that these patients with HRp tumors will most likely be resistant to platinum chemotherapy and in need of maintenance therapy, so niraparib would provide them with a longer PFS anyway.
Patients with an HRd-positive BRCA mutation remain the patients who stand to benefit the most from niraparib monotherapy. According to Hamilton, data presented at the 2023 American Society of Clinical Oncology annual meeting showed that a BRCA mutation and the patient’s HRd status are predictors of long-term PFS on niraparib.18 An analysis of results from the PRIMA trial showed those patients with BRCA mutation and HRd-positive tumors had an odds ratio (OR) of 3.73 (95% CI, 2.19-6.32) for a PFS rate of 2 years or greater, while patients with BRCA1-mutated tumors had an OR of 10.75 and those with a BRCA2 mutation had an OR of 2.49 (P < .0001). This bolstered his reasoning for providing niraparib monotherapy if the patient is eligible for it.
“All patients on a PARP inhibitor will have a treatment-emergent adverse event and encounter dosage reductions or interruptions,” explained Hamilton when reminding physicians about the safety profile of niraparib. “The thing we’ve learned with niraparib is that it’s a dose-finding exercise in those first 2 to 3 months of treatment, but after that, most of those adverse events are not an issue during treatment and dissipate over time.”
References
1. Key statistics for ovarian cancer. American Cancer Society. Updated January 12, 2023. Accessed August 28, 2023. https://tinyurl.com/yc59t68c
2. SEER Cancer Statistics Review (CSR) 1975-2017. National Cancer Institute Surveillance, Epidemiology, and End Results Program. April 15, 2020. Accessed August 28, 2023. https://tinyurl.com/37nfstcx
3. BRCA gene mutations: cancer risk and genetic testing. National Cancer Institute. Updated November 19, 2020. Accessed August 29, 2023. https://tinyurl.com/46s2knv2
4. Targeted drug therapy for ovarian cancer. American Cancer Society. Updated November 17, 2022. Accessed August 29, 2023. https://tinyurl.com/y8xz7d5n
5. Ginolhac SM, Gad S, Corbex M, et al. BRCA1 wild-type allele modifies risk of ovarian cancer in carriers of BRCA1 germ-line mutations. Cancer Epidemiol Biomarkers Prev. 2003;12(2):90-95.
6. Fuh K. Mismatch repair deficiency in ovarian cancer. Gynecol Oncol Rep. 2022;41:101015. doi:10.1016/j.gore.2022.101015
7. Mismatch repair deficiency and MSI. Genomics Education Programme. Update March 25, 2022. Accessed August 29, 2023. https://tinyurl.com/yzy6vw9a
8. Konstantinopoulos PA, Norquist B, Lacchetti C, et al. Germline and somatic tumor testing in epithelial ovarian cancer: ASCO guideline. J Clin Oncol. 2020;38(11):1222-1245. doi:10.1200/JCO.19.02960
9. Creeden JF, Nanavaty NS, Einloth KR, et al. Homologous recombination proficiency in ovarian and breast cancer patients. BMC Cancer. 2021;21(1):1154. doi:10.1186/s12885-021-08863-9
10. Arora S, Balasubramaniam S, Zhang H, et al. FDA approval summary: olaparib monotherapy or in combination with bevacizumab for the maintenance treatment of patients with advanced ovarian cancer. Oncologist. 2021;26(1):e164-e172. doi:10.1002/onco.13551
11. FDA approves rucaparib for maintenance treatment of recurrent ovarian, fallopian tube, or primary peritoneal cancer. News release. FDA. April 6, 2018. Accessed August 29, 2023. https://tinyurl.com/3um89k76
12. FDA approves niraparib for first-line maintenance of advanced ovarian cancer. News release. FDA. April 29, 2020. Accessed August 29, 2023. https://tinyurl.com/web9fshp
13. Vergote I, Denys H, De Greve J, et al. Treatment algorithm in patients with ovarian cancer. Facts Views Vis Obgyn. 2020;12(3):227-239.
14. Csizmar CM, Saliba AN, Swisher EM, Kaufmann SH. PARP inhibitors and myeloid neoplasms: a double-edged sword. Cancers (Basel). 2021;13(24):6385. doi:10.3390/cancers13246385
15. Lorusso D, Mouret-Reynier M-A, Harter P, et al. 5-year (y) overall survival (OS) with maintenance olaparib (ola) plus bevacizumab (bev) by clinical risk in patients (pts) with newly diagnosed advanced ovarian cancer (AOC) in the phase III PAOLA-1/ENGOT-ov25 trial. ESMO Open. 2023;8(supp 1):100812. doi:10.1016/j.esmoop.2023.100812
16. González-Martín A, Pothuri B, Vergote I, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391-2402. doi:10.1056/NEJMoa1910962
17. González-Martín A, Pothuri B, Vergote I, et al. Progression-free survival and safety at 3.5years of follow-up: results from the randomised phase 3 PRIMA/ENGOT-OV26/GOG-3012 trial of niraparib maintenance treatment in patients with newly diagnosed ovarian cancer. Eur J Cancer. 2023;189:112908. doi:10.1016/j.ejca.2023.04.024
18. Graybill W, Pardo B, O’Malley DM, et al. Predictors of long-term progression-free survival (PFS) in niraparib-treated patients (pts) from the PRIMA/ENGOT-OV26/GOG-3012 study. J Clin Oncol. 2023;41(suppl 16):5589. doi:10.1200/JCO.2023.41.16_suppl.5589

Advances in Subsequent Therapies Shake Up Sequencing of ccRCC Treatment
April 25th 2024With the approval of belzutifan and other newer data for treating patients with recurrent renal cell carcinoma, the state of subsequent therapies is advancing beyond the reuse of frontline options with impacts on duration of response and quality of life.
Read More
Novel Approaches Focus on Limiting Toxicity in Older Patients With ALL
April 22nd 2024The major challenges for clinicians treating older patients with acute lymphoblastic leukemia surround the emergence of resistance to existing therapies and the toxicities associated with current chemotherapies.
Read More
Ornstein Advises on Starting Dose and Management of Lenvatinib in RCC
April 21st 2024During a Case-Based Roundtable® event, Moshe Ornstein, MD, MA, provided guidance on dosing and toxicity concerns in a patient treated with lenvatinib plus pembrolizumab for advanced renal cell carcinoma.
Read More