Understanding the Checkpoint Inhibitor-Resistant Disease State
Metastatic urothelial cancer is a relatively chemotherapy-sensitive malignancy. With contemporary cisplatin-based combination chemotherapy regimens, objective responses are achieved in approximately 50-60% of patients and complete radiographic responses are achieved in approximately 10-20% of patients.
Matthew D. Galsky, MD
Metastatic urothelial cancer is a relatively chemotherapy-sensitive malignancy. With contemporary cisplatin-based combination chemotherapy regimens, objective responses are achieved in approximately 50-60% of patients and complete radiographic responses are achieved in approximately 10-20% of patients.1Unfortunately, response durations are typically short with only 5-10% of patients achieving durable disease control. Decades of clinical trials exploring modifications of cytotoxic regimens, including the use of different or more drugs, demonstrated that a therapeutic plateau had been reached with conventional chemotherapeutic agents, highlighting the need for novel approaches.
Recently, immune checkpoint blockade has demonstrated proof of concept in the clinic and has been rapidly integrated into standard care for the treatment of advanced urothelial cancer. While immune checkpoint blockade has changed the landscape of treatment of advanced urothelial cancer, the majority of patients don’t respond to such treatment. This creates a new clinical disease statecancer that is “immune-checkpoint blockade resistant.” Herein, I outline the current knowledge regarding immune checkpoint blockade in the treatment of urothelial cancer as a basis of framing my presentation this afternoon on treatment of patients progressing despite such treatment.
In the initial phase I studies exploring PD-1 and PD-L1 antibodies, it was apparent early on that the spectrum of anticancer activity was broader than had been the experience from CTLA-4 blockade. Objective responses were seen in cancers commonly considered “immune-responsive,” such as melanoma and renal cancer, but also somewhat unexpectedly in other solid tumors such as nonsmall cell lung cancer.2,3These findings, as well as data demonstrating that tumor-infiltrating lymphocytes are prognostic in urothelial cancer specimens, provided the impetus to expand phase I cohorts to include urothelial cancer. Initial proof-of-concept for PD-L1 and PD-1 blockade in urothelial cancer came from expansion cohorts of phase I studies of atezolizumab (Tecentriq) and pembrolizumab (Keytruda), respectively.4,5Both of these studies demonstrated durable objective responses in a subset of heavily pretreated patients with metastatic urothelial cancer, with a generally favorable side effect profile compared with patients historically treated with cytotoxic chemotherapy, serving as the basis for much more extensive investigation.
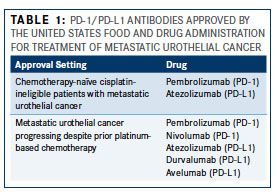
As of May 2017, two PD-1 inhibitors (pembrolizumab and nivolumab [Opdivo]) and 3 PD-L1 inhibitors (atezolizumab, durvalumab [Imfinzi], and avelumab [Bavencio]) have been approved by the FDA for the treatment of metastatic urothelial cancer (Table 1). The datasets leading to approval of these therapies range from expansion cohorts of phase I studies to large randomized phase III studies and are summarized in Table 2. In patients with metastatic urothelial cancer progressing despite prior platinum-based chemotherapy, these trials have demonstrated objective response rates (ORRs, complete responses + partial responses) of approximately 15-20%. Although, unfortunately, the vast majority of patients with metastatic urothelial cancer do not respond to PD-1/PD-L1 blockade, responses that do occur tend to be durable. That is, in most trials reported to date, the median duration of response had not been reached at the time the data was locked for analysis with responses routinely lasting >1-2 years.
Two phase III trials have now been reported randomizing patients with metastatic urothelial cancer progressing despite platinum-based chemotherapy to PD-1/PD-L1 blockade versus “standard of care” chemotherapy (eg, docetaxel). Keynote-045 randomized 542 patients to receive either pembrolizumab or chemotherapy with a co-primary endpoint of progression-free survival (PFS) and overall survival (OS).6This trial demonstrated a significant improvement in ORR (21.1% vs 11.4%) and median OS (10.3 months vs 7.4 months) with pembrolizumab whereas there was no significant difference in PFS. IMvigor 211 was a larger, although similarly designed study, randomizing 931 patients with metastatic urothelial cancer progressing despite prior platinum-based chemotherapy to atezolizumab versus chemotherapy.7This trial utilized a hierarchical statistical analysis which required that atezolizumab demonstrate an OS benefit in the subset of patients with the highest expression of PD-L1 on tumor-infiltrating cells in archival tumor specimens prior to extending the statistical analysis to the entire trial population. Unfortunately, a survival benefit was not demonstrated in the biomarker-defined population impairing the ability to interpret the study overall. Of note, in this large phase III study, atezolizumab performed similarly to the results previously reported in the phase II IMvigor 210 study of atezolizumab.
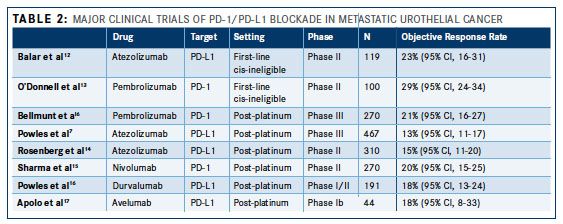
In addition to the platinum-resistant metastatic setting, 2 large single-arm phase II trials have also demonstrated the activity of PD-1/PD-L1 blockade as first-line treatment for cisplatin-ineligible patients with metastatic urothelial cancer. Athough cisplatin-based chemotherapy is standard first-line treatment for patients with metastatic urothelial cancer, a large proportion of patients are deemed “cisplatin- ineligible” due to borderline performance status and/or comorbidities such as renal impairment, hearing loss, or peripheral neuropathy.8,9Carboplatin-based chemotherapy has typically been administered in this setting but is associated with inferior outcomes compared with cisplatin-based combinations and is associated with an unfavorable toxicity profile.10,11Therefore, PD-1/PD-L1 blockade has been explored in this setting given the unmet need. In the IMvigor 210 study, atezolizumab was explored in a cohort of 119 chemotherapy naïve cisplatin-ineligible patients with metastatic urothelial cancer yielding a response rate of 23% (95% CI, 16-31).12Keynote-052 was a similarly designed trial, enrolling 270 patients, and reporting an ORR of 29% (95% CI, 24-34).13As demonstrated in trials exploring patients with cisplatin-resistant metastatic urothelial cancer, these responses were generally prolonged, leading to integration of such treatment as a standard option for chemotherapy naïve cisplatin-ineligible patients.
With immune checkpoint blockade now occupying a role in the standard treatment of metastatic urothelial cancer in both the first-line (cisplatin-ineligible) and second-line treatment settings, there is a growing population of patients who have previously been exposed to such therapies. Promising approaches in the immune checkpoint blockade-resistant setting include chemotherapy plus anti-angiogenic therapy, antibody-drug conjugates, and small-molecule-targeted recurrent molecular alterations in urothelial cancer.
References:
- Bellmunt J, von der Maase H, Mead GM, et al. randomized phase III study comparing paclitaxel/cisplatin/ gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC intergroup study 30987. J Clin Oncol. 2012;30(10):11071113. doi: JCO.2011.38.6979 [pii] 10.1200/JCO.2011.38.6979.
- Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):316775. doi: 10.1200/JCO.2009.26.7609.
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti pd-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690.
- Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558562. doi: 10.1038/ nature13904.
- Plimack ER, Bellmunt J, Gupta S, et al. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE-012): a non-randomised, open-label, phase 1b study. Lancet Oncol. 2017;18(2):212220. doi: 10.1016/S1470- 2045(17)30007-4.
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017:NEJMoa1613683. doi: 10.1056/NEJMoa1613683.
- Powles T, Loriot Y, Duran I, et al. IMvigor 211: A phase III randomized study examining atezolizumab vs. chemotherapy for platinum-treated advanced urothelial cancer. Presented at: EACR-AACR-SIC Special Conference 2017; June 24-27, 2017; Florence, Italy. Abstract 606.
- Galsky MD, Hahn NM, Rosenberg J, et al. A consensus definition of patients with metastatic urothelial carcinoma who are unfit for cisplatin-based chemotherapy. Lancet Oncol. 2011;12(3):211214. doi: S1470-2045(10)70275-8 [pii] 10.1016/S1470- 2045(10)70275-8.
- Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol. 2011;29(17):24322438. doi: JCO.2011.34.8433 [pii] 10.1200/JCO.2011.34.8433.
- Galsky MD, Chen GJ, Oh WK, et al. Comparative effectiveness of cisplatin-based and carboplatin- based chemotherapy for treatment of advanced urothelial carcinoma. Ann Oncol. 2011;23(2):406410. doi: mdr156 [pii] 10.1093/annonc/mdr156.
- De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/ carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30(2):191199. doi: JCO.2011.37.3571 [pii] 10.1200/JCO.2011.37.3571.
- Balar A V, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin- ineligible patients with locally advanced and metastatic urothelial carcinoma: a single- arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):6776. doi: 10.1016/S0140- 6736(16)32455-2.
- O’Donnell PH, Grivas P, Balar AV, et al. Biomarker findings and mature clinical results from KEYNOTE-052: first-line pembrolizumab (pembro) in cisplatin-ineligible advanced urothelial cancer (UC). J Clin Oncol. 2017;35(15_suppl):4502. doi: 10.1200/ JCO.2017.35.15_suppl.4502.
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet (London, England). 2016. doi: 10.1016/S0140-6736(16)00561-4.
- Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017. doi: 10.1016/S1470-2045(17)30065-7.
- Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma. JAMA Oncol. 2017;45(2):e172411. doi: 10.1001/jamaoncol.2017.2411.
- Apolo AB, Infante JR, Balmanoukian A, et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, phase ib study. J Clin Oncol. 2017:JCO2016716795. doi: 10.1200/ JCO.2016.71.6795.