Tweet Chat Recap: Selecting Treatment for a HER2-Positive Metastatic Breast Cancer Case Scenario
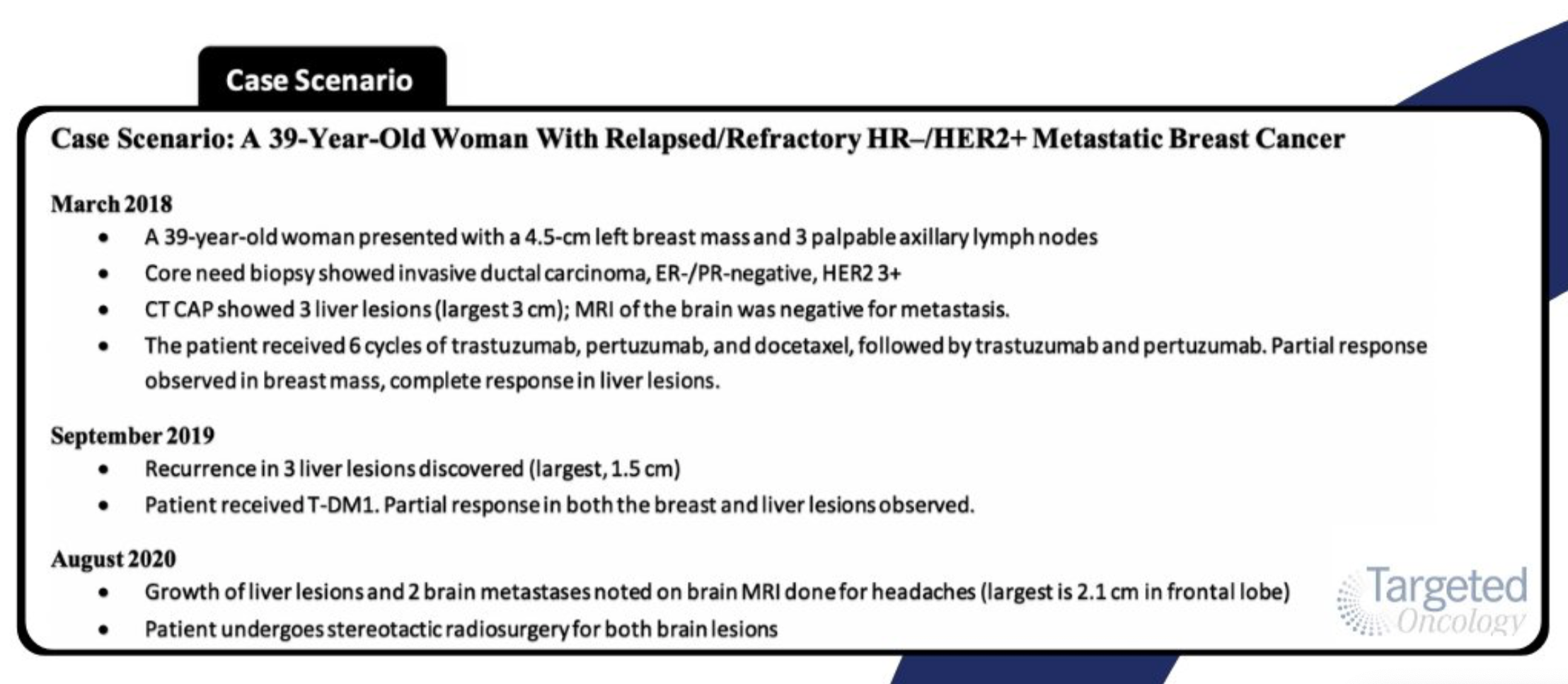
During a recent tweet chat, Sara M. Tolaney, MD, MPH, joined Targeted Oncology for the discussion of a 39-year-old woman with relapsed/refractory HR-negative/HER2-positive metastatic breast cancer.
Sara M. Tolaney, MD, MPH

The treatment landscape for HER2-positive metastatic breast cancer has expanded as a result of recent data from randomized clinical trials. Experts are finding that each case of HER2-positive metastatic breast cancer is slightly different, therefore, requiring a more precise approach for treatment.
During a recent tweet chat, Sara M. Tolaney, MD, MPH, associate director of the Susan F. Smith Center for Women’s Cancers; director of Clinical Trials, Breast Oncology; and senior physician at Dana-Farber Cancer Institute, and assistant professor of medicine at Harvard Medical School, joined Targeted Oncology for the discussion of a 39-year-old woman with relapsed/refractory HR-negative/HER2-positive metastatic breast cancer.
In the tweet chat case scenario, the patient was initially treated with 6 cycles of trastuzumab (Herceptin), pertuzumab (Perjeta), and docetaxel, followed by trastuzumab and pertuzumab. Despite a partial response (PR) was observed in the breast mass and a complete response (CR) in the liver lesions, the patient recurred 18 months later and was treated with trastuzumab emtansine (T-DM1; Kadcyla), at which time she achieved a PR in the breast and liver lesions. About 1 year later, the patient returned to the clinic with growth of the liver lesions and 2 brain metastases. To address the liver size increase and metastases in the brain, the patients underwent stereotactic radiosurgery (SRS) for both brain lesions.
In a twitter poll ahead of the chat, we shared the tweet chat case scenario and asked what physicians would recommend as the next best line of therapy for this patient. The options included tucatinib (Tukysa), capecitabine, and trastuzumab; trastuzumab deruxtecan (Enhertu), neratinib (Nerlynx) and capecitabine, or other. The triplet regimen of tucatinib with capecitabine and trastuzumab appeared to be the most preferred therapeutic approach for this patient given her history.
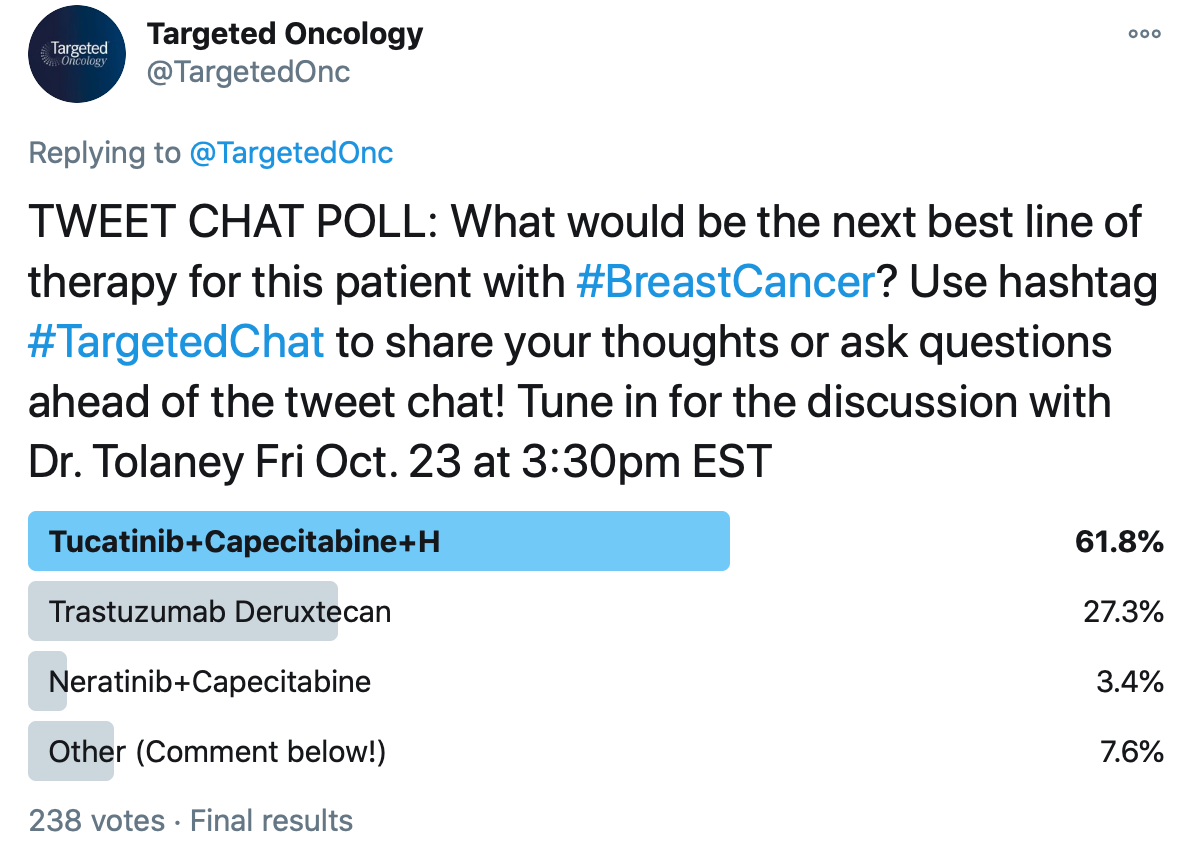
A total of 238 votes were gathered in this poll, which favored the tucatinib regimen, which received its FDA approval earlier this year based on the findings from the phase 2 HER2CLIMB clinical trial (NCT02614794). Jafar Al-Mondhiry, MD, MA, hematology/oncology fellow, University of California Los Angeles (UCLA), tweeted, “Tucatinib for the CNS [central nervous system] benefits, hands down.”
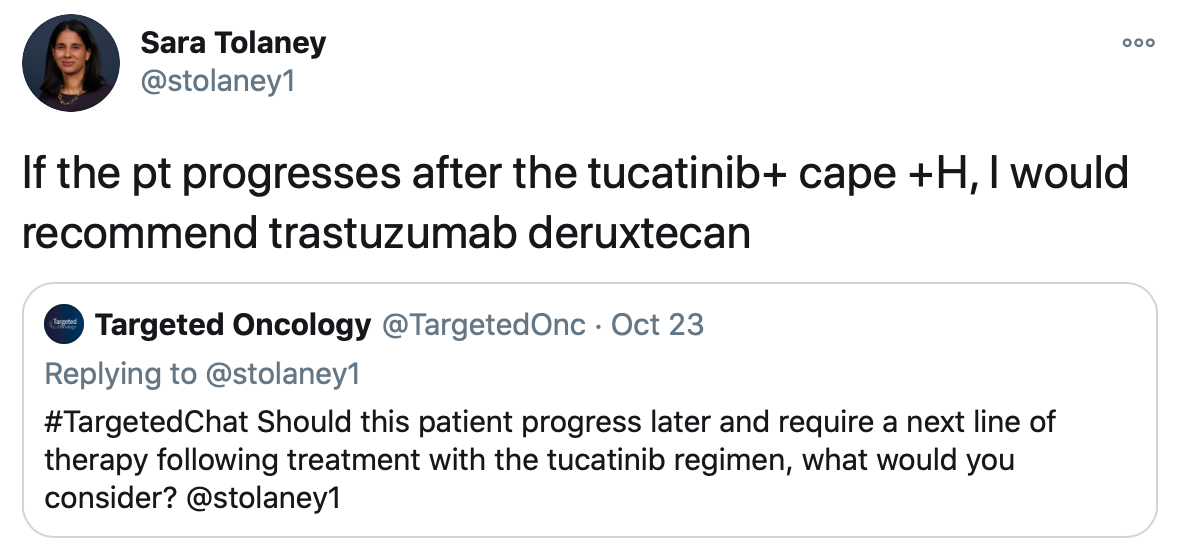
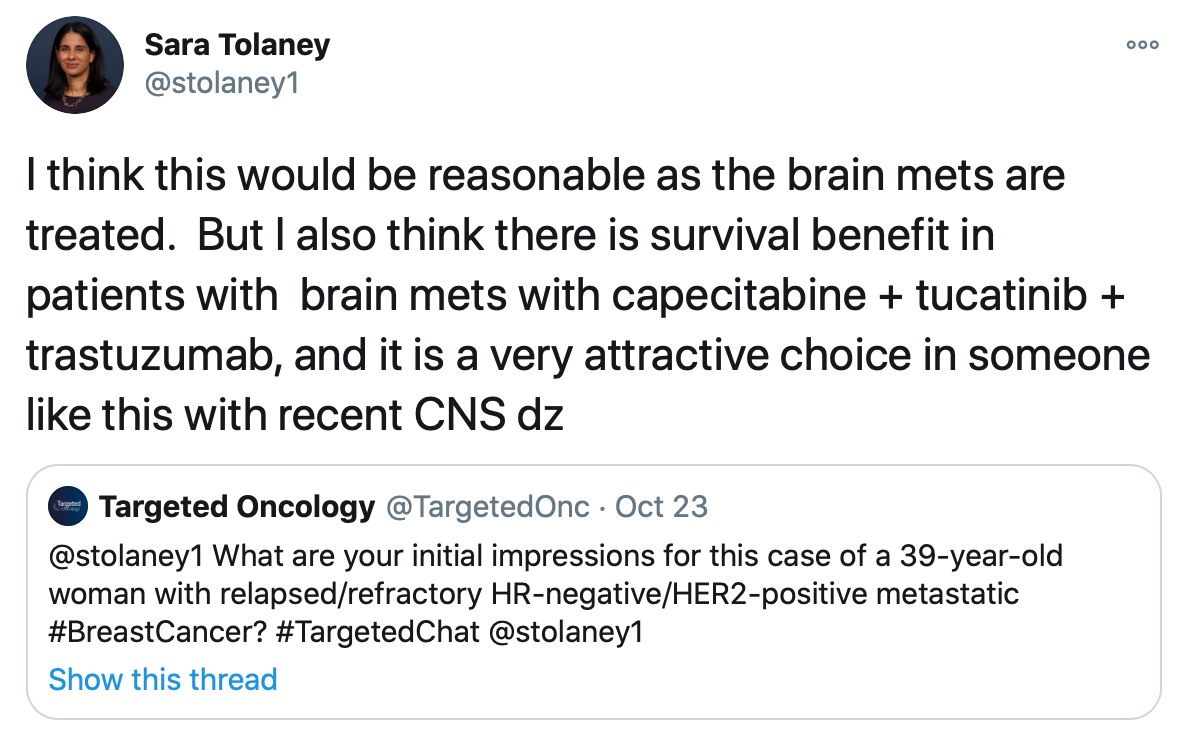
During the tweet chat, Tolaney prioritized the tucatinib regimen and trastuzumab deruxtecan as her 2 top options for this patient over the neratinib/capecitabine treatment. She tweeted that she thinks either of these treatments would be appropriate considering the data supporting both.
“Data from HER2CLIMB found a significant improvement in PFS [progression-free survival] and OS [overall survival], and this included patients with stable treated brain mets, as well as active brain mets,” Tolaney tweeted during the discussion.
In order to make the decision between these 2 potential treatment approaches, Tolaney told Targeted Oncology in an interview following the tweet chat discussion, that physicians should consider the efficacy and safety data.
HER2CLIMB Data Supporting the Tucatinib Regimen
Tucatinib in combination with trastuzumab and capecitabine is indicated for the treatment of adult patients with advanced unresectable or metastatic HER2-positive breast cancer, which includes those with brain metastases who have received at least 1 previous anti-HER2-based regimen in the metastatic setting. The supportive data from the HER2CLIMB study were published in the New England Journal of Medicine in 2020.1,2
“In this study, we saw that adding tucatinib to trastuzumab and capecitabine not only improved PFS, but it also improved OS,” Tolaney said. “This OS benefit was seen in patients not only with just systemic disease but also in patients with active brain metastases. To me, that is very compelling because we have never had a drug yet that has improved survival for patients with active brain metastases.”
The findings showed statistically significant clinically meaningful improvements in both PFS and OS with the triplet regimen in patients with HER2-positive metastatic breast cancer, irrespective of brain metastases. The median OS with tucatinib was 21.9 months (95% CI, 18.3-31.0) compared with 17.4 months in the placebo arm (95% CI, 13.6-19.9). The risk of death was reduced by 34% in the overall population (HR, 0.66; 95% CI, 0.50-0.88; P =.005).
The median duration of exposure to the tucatinib regimen was 7.3 months (range, <0.1-35.1) and 4.4 months with the placebo regimen (range, <0.1-24.0). The risk of progression or death was reduced by 46% (HR, 0.54; 95% CI, 0.42-0.71; P <.001) overall and 52% (HR, 0.48; 95% CI, 0.34-0.69; P <.001) among those with brain metastases.
The most common adverse events (AEs) observed in the tucatinib arm versus control arm, respectively, included diarrhea (80.9% vs 53.3%), palmar-plantar erythrodysesthesia syndrome (63.4% vs 52.8%), nausea (58.4% vs 43.7%), fatigue (45.0% vs 43.1%), and vomiting (35.9% vs 25.4%).
Co-investigator of HER2CLIMB, Sarah A. Hurvitz, MD, of the UCLA Medical Center, previously commented on the relevance of trastuzumab deruxtecan in this patient population, even with the new data for the HER2CLIMB regimen, “Although the data relating to tucatinib, capecitabine, and trastuzumab are very exciting and indeed practice-changing, we also have the FDA approval of trastuzumab deruxtecan, which is also incredibly effective for patients.”
DESTINY-Breast01 Findings Supporting Trastuzumab Deruxtecan
Trastuzumab deruxtecan received an accelerated approval in December 2019 for the treatment of patients with unresectable or metastatic HER2-positive breast cancer following at least 2 prior lines of anti-HER2-based regimens in the metastatic setting. This agent received its approval based on the findings from the phase 2 DESTINY-Breast01 study, which were presented during the 2019 San Antonio Breast Cancer Symposium (SABCS) and late published in the New England Journal of Medicine.3,4
The study demonstrated an objective response rate (ORR) of 60.9% per Independent Central Review (95% CI, 53.4-68.0), which included a 6.0% CR rate and a 54.9% PR rate, and 36.4% of patients had stable disease, while 3 had progressive disease. The ORRs were consistent across subgroups of patients, including those with prior treatment with pertuzumab and those with at least 3 prior therapies, similar to the patient in the tweet chat case scenario.
The disease control rate was 97.3% (95% CI, 93.8-99.1), and the clinical benefit rate was 76.1% (95% CI, 69.3-82.1).
The HER2-targeted antibody-drug conjugate induced a median duration of response (DOR) of 14.8 months (95% CI, 13.8-16.9) and a disease control rate of 97.3% (95% CI, 93.8-99.1). Overall, the median PFS was 16.4 months (95% CI, 12.7-not evaluable [NE]), and the median PFS among those with brain metastases was 18.1 months (95% CI, 6.7-18.1). The median OS was not reached (95% CI, NE-NE).
"If you look at the median PFS in HER2CLIMB, it's around 8 months, but if you look at trastuzumab deruxtecan, the median is [around] 16 months in patients with a median of 6 prior lines of therapy," Tolaney said.
"This is really not something we ever see, but on the flip side, it is just a single-arm phase 2 study. It did allow patients with active brain metastases, and it does have the risk of interstitial lung disease (ILD), whereas tucatinib does certainly have extra toxicities in terms of some diarrhea and some elevated liver function tests, but there were a couple patients who actually died from ILD in the DESTINY-Breast01 study. I think it's something we keep in mind when trying to make treatment decisions,” she added.
ILD was experienced by 13.6% of patients, but it was primarily grade 1 (2.7%) or grade 2 (8.2%). The median time to onset was 193 days (range, 42-535), and corticosteroids were used in 13 of 20 patients with grade ≥2 ILD. In total, 7 patients recovered from ILD, 2 were recovering at the data cutoff, 12 had an unknown outcome or were not followed until resolution, and 4 died.
TRAEs were observed in almost all patients (99%), but only around half (51%) of patients experienced TRAEs of grade 3 or worse severity. The most common any-grade TEAEs were nausea (77%), alopecia (48%), fatigue (48%), vomiting (45%), constipation (34%), decreased neutrophil count (31%), decreased appetite (29%), diarrhea (27%), and anemia (26%), while the most common grade ≥ 3 AEs included decreased neutrophil count (31%), nausea (7.6%), and anemia (in 26%).
Although these data are encouraging for trastuzumab deruxtecan, Tolaney ultimately selected tucatinib with trastuzumab and capecitabine as her recommendation for the next line of therapy.
"You have a choice here, and I would consider the toxicity risk and the fact that the patient just had her brain mets treated, so she’s not truly stable in a sense. We haven't followed this patient, so I think given those 2 factors, toxicity and [stability] of the brain mets, I probably would favor the HER2CLIMB regimen in this particular patient.”
Although trastuzumab deruxtecan wouldn’t be her preferred next line, Tolaney still spoke to where this therapy could fit into the treatment landscape for this patient. When asked what she would do if the patient had progressed on the tucatinib triplet, Tolaney would then opt for trastuzumab deruxtecan.
Neratinib Plus Capecitabine Findings from NALA Study
The combination of neratinib and capecitabine was also approved this year by the FDA for the treatment of patients with advanced or metastatic HER2-positive breast cancer who have received at least 2 prior anti-HER2-based regimens in the metastatic setting. The approval was based on findings from the randomized open-label multicenter phase 3 NALA study (NCT01808573), which demonstrated a 24% reduction in the risk of disease progression or death in comparison with lapatinib (Tykerb) and capecitabine.5
The median PFS with neratinib was 8.8 months compared with 6.6 months in the control arm (HR, 0.76; 95% CI, 0.63-0.93; stratified log-rank P =.0059). The median OS was 21 months (95% CI, 17.7-23.8) for the neratinib combination versus 18.7 months with the lapatinib regimen (95% CI, 15.5-21.2) in the NALA study (HR, 0.88; 95% CI, 0.72-1.07; stratified log-rank P =.2086).6
The ORR was 32.8% (95% CI, 27.1%-38.9%) with neratinib versus 26.7% with lapatinib (95% CI, 21.5%-32.4%), and the median DOR was 8.5 months (95% CI, 5.6-11.2) versus 5.6 months (95% CI, 4.2-6.4), respectively.
The most common treatment-emergent AEs of any grade included diarrhea (83.2%), nausea (53.1%), palmar-plantar erythrodysesthesia syndrome (45.9%), and vomiting (45.5%). The investigators of NALA noted that grade 3 diarrhea was observed in 24.4% of patients in the neratinib arm versus 12.5% in the lapatinib arm, and most patients experienced this event during the first cycle of treatment.
“I think the challenge for me is that neratinib plus capecitabine did not have a survival benefit, whereas HER2CLIMB did meet a survival benefit,” Tolaney said. “Despite the use of prophylactic loperamide in NALA, we did still see a little over 20% rate of grade 3/4 diarrhea, and that's certainly much higher than we saw in HER2CLIMB. Because of the increased toxicity and lack of survival benefit, I tend to choose the tucatinib/trastuzumab/capecitabine regimen over capecitabine with neratinib.”
Presence of Brain Metastases Impacts Treatment Decisions
In the tweet chat case scenario, the patient presented with 2 brain metastases, and so the patient underwent SRS. This ultimately played a big part in the treatment decision-making process for Tolaney, in which she favored tucatinib in combination with trastuzumab and capecitabine over trastuzumab deruxtecan. Although the brain metastases were treated with SRS, the triplet regimen was more favorable considering the recent CNS in this patient.
In a Twitter poll, Targeted Oncology asked whether or not physicians agreed with this decision for treating the brain metastases in this tweet chat case scenario. The majority (66.7%) agreed with use of SRS, which they would have followed with a tyrosine kinase inhibitor (TKI), while 22.2% would have used SRS alone. The 2 remaining options included in this poll considered whole-brain radiotherapy (WBRT) and use of a TKI without SRS.
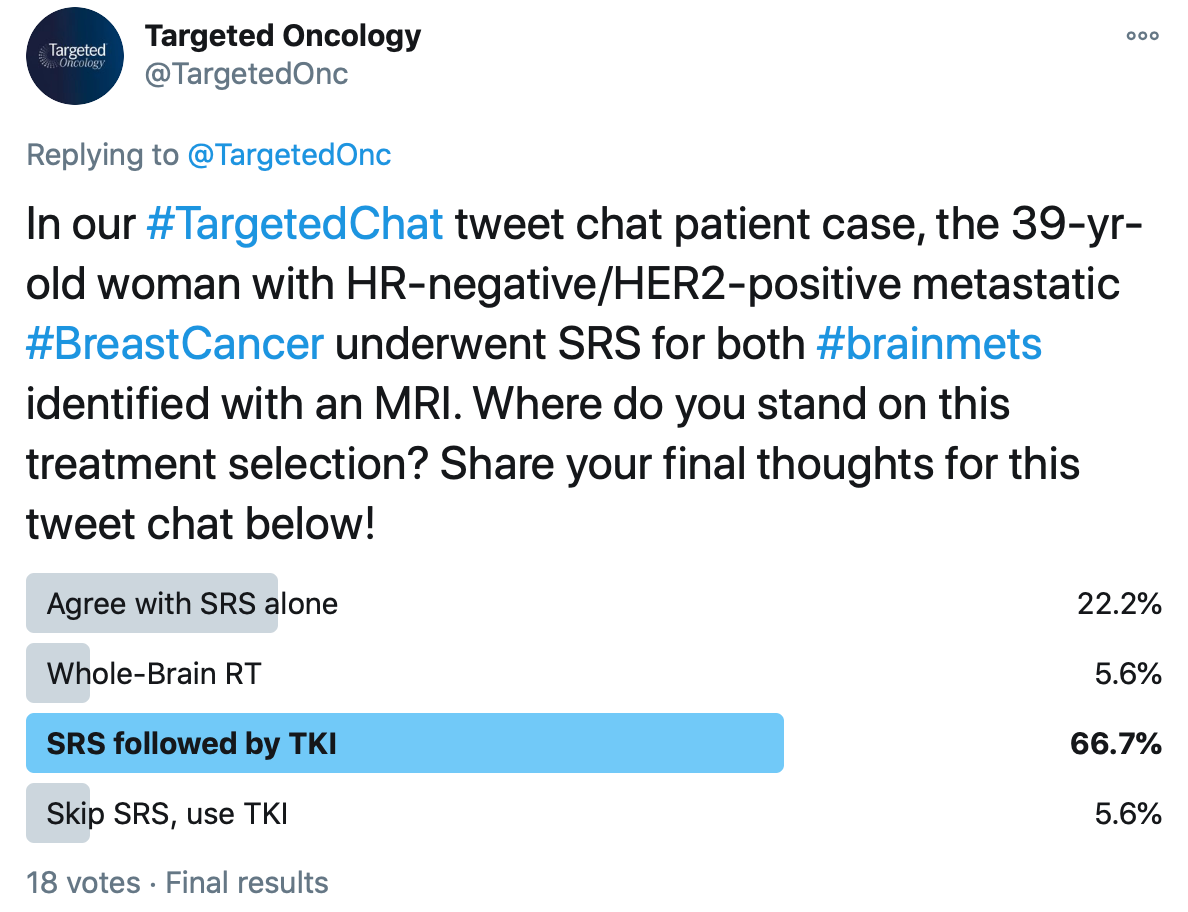
“When someone presents with CNS involvement with HER2-positive disease, we're usually looking to think about doing local therapy with radiation, and that has been our standard to date,” Tolaney said. “If a patient has disease that could be amenable to SRS, our preference is usually to use SRS to minimize toxicity and spare that patient WBRT. That usually would be my preference, and so in this particular case, I would have also moved forward with SRS.”
Although Tolaney agrees with the decision to use SRS in this patient, she said that this brings up an important question of whether physicians could skip SRS and move immediately to the tucatinib, trastuzumab, and capecitabine regimen. In the HER2CLIMB study, patients with untreated brain metastases were included if they were asymptomatic, serving as supportive data for this potential approach to brain metastases.
“It is an option for patients to omit radiation and get the HER2CLIMB regimen,” Tolaney said. “They could then potentially reserve radiation for the future if they were to develop CNS progression, so that would also be a fair choice in this particular situation.”
An exploratory analysis of HER2CLIMB demonstrated multiple positive responses with the triplet regimen in patients with HER2-positive metastatic breast cancer with brain metastases. These data were presented during the 2020 Society for Neuro-Oncology (SNO) Conference on Brain Metastases by Nancy Lin, MD, associate chief, Division of Breast Oncology, Susan F. Smith Center for Women's Cancers; director, Metastatic Breast Cancer Program; senior physician, Dana-Farber Cancer Institute; and associate professor of medicine, Harvard Medical School.7
“The important distinction in these patients, in addition to the high proportion within the trial overall, is that 60% of the patients with brain metastases enrolled had active brain metastases,” Lin told Targeted Oncology, in an interview following the conference. “For the vast majority, if not in all registration trials in breast cancer, if they've included patients with brain metastases, they have only those with treated and stable brain metastases. This study is the first registration trial of breast cancer to include patients with active brain metastases.”
Among patients with active brain metastases in HER2CLIMB, the CNS-PFS and OS benefits were maintained, in which the median CNS-PFS was 9.9 months versus 4.2 months with placebo (HR, 0.32; 95% CI, 0.22-0.48; P <. 00001), and the median OS was extended by more than 9 months with tucatinib, which was 20.7 months versus 11.6 months with placebo (HR, 0.49; 95% CI, 0.30-0.80; P =.004).
The CNS-PFS benefit was also maintained among patients with stable brain metastases who received tucatinib versus placebo, in which the median CNS-PFS was 13.9 months versus 5.6 months, respectively, (HR, 0.31; 95% CI, 0.14-0.67; P =.002).
“Given that the overall population of patients [in HER2CLIMB] include those with and without brain metastases, I think that these findings overall firmly established the use of tucatinib in combination with trastuzumab and capecitabine for the treatment of HER2-positive metastatic breast cancer,” Lin said.
Remaining Unmet Needs to Address
Although the use of tucatinib with trastuzumab deruxtecan and capecitabine appears most promising in this setting, the role of trastuzumab deruxtecan in patients with CNS remains unknown.
"The trial exploring trastuzumab deruxtecan does not address what to do for patients with active CNS disease. However, it doesn't mean it's not active in the CNS," said Tolaney. "I think it does mean that we need more data, and so there is work ongoing trying to address if trastuzumab deruxtecan has activity in the CNS.”
The phase 2 DEBBRAH clinical trial (NCT04420598) is currently recruiting and will explore the use of trastuzumab deruxtecan as treatment of patients with active brain metastases. This is a multicenter, international, open-label, single-arm study which will utilize a 2-stage optimal Simon’s design. There will be 5 cohorts of patients, which will include those with HER2-positive breast cancer with non-progressing brain metastases after WBRT, SRS, or surgery (cohort 1); HER2-positive or -low disease with asymptomatic, untreated brain metastases (cohort 2); HER2-positive breast cancer with progressing brain metastases after local treatment (cohort 3); her2-low expressing disease with progressing brain metastases after local therapy (cohort 4); and HER2-positive or -low expressing breast cancer with leptomeningeal carcinomatosis.
The primary end point is PFS at 16 weeks for cohort 1, CNS ORR for cohorts 2, 3, and 4, and OS for cohort 5.
Overall, this study will help investigators determine the role of trastuzumab deruxtecan as treatment of patients with HER2-positive metastatic breast cancer who have brain metastases. As this trial and similar studies continue to roll out, physicians will have more definitive answers in terms of how to treat patients with HER2-positive breast cancer and active brain metastases.
<<Check out more on the evolving role of tucatinib in this Investigator Perspectives series
References
1. Seattle Genetics announces U.S. FDA approval of Tukysa (tucatinib) for people with advanced unresectable or metastatic HER2-positive breast cancer. News Release. Seattle Genetics, Inc. April 17, 2020. Accessed October 26, 2020. https://bit.ly/2xtQeBe
2. Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med. 2020;382(7):597-609. doi: 10.1056/NEJMoa1914609
3. FDA approves new treatment option for patients with HER2-positive breast cancer who have progressed on available therapies. Press Release. December 20, 2019. Accessed October 26, 2020. https://bit.ly/2tzcabv
4. Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020; 382(7):610-621. doi: 10.1056/NEJMoa1914510
5. Puma Biotechnology receives U.S. FDA approval of supplemental New Drug Application for neratinib to treat HER2-positive metastatic breast cancer. News Release. Puma Biotechnology, Inc. February 26, 2020. Accessed October 27, 2020. https://bit.ly/2TfqDmc.
6. Saura C, Oliveira M, Feng YH, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: phase iii NALA trial. J Clin Oncol. Published online July 17, 2020. doi: 10.1200/JCO.20.00147
7. Lin MU, Borges V, Anders C, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020;38:(23):2610-261. doi: 10.1200/JCO.20.00775
Durability and Intracranial Efficacy Observed With T-DXd in HER2+ MBC
January 13th 2025During a Case-Based Roundtable® event, Ian Krop, MD, and participants discussed how the outcomes of the DESTINY-Breast03 and other trials impact treatment of metastatic HER2-positive breast cancer in the second article of a 2-part series.
Read More