Tweet Chat Recap: Evolving Options for Newly Diagnosed Multiple Myeloma
During a recent tweet chat with Alexander M. Lesokhin, MD, a hematologic oncologist with Memorial Sloan Kettering Cancer Center, joined Targeted Oncology to discuss the case of a 72-year-old White man with newly-diagnosed multiple myeloma.
Alexander M. Lesokhin, MD

The treatment landscape for newly- diagnosed multiple myeloma has evolved rapidly in recent years, with new treatment options and studies supporting different regimens. Each regimen has its own benefits and drawbacks when it comes to efficacy and toxicity.
During a recent tweet chat with Alexander M. Lesokhin, MD, a hematologic oncologist with Memorial Sloan Kettering Cancer Center, joined Targeted Oncology to discuss the case of a 72-year-old White man with newly-diagnosed multiple myeloma.
During the scenario, the man presented to his primary care physician complaining of fatigue. The patient has a medical history of osteoarthrosis and limited mobility. A physical exam reviled pallor, hypertrophic changes at distal and proximal interphalangeal joints, poor grip strength, and bilateral swelling in shoulder joints. Blood work found anemia (hemoglobin: 10.2g/dL), hypercalcemia (corrected calcium: 12.9 mg/dL), elevated creatinine (1.5 mg/dL), and a creatinine clearance of 50mL/min.
Blood tests showed the patient had a hemoglobin level of 10.3g/dl, myeloma protein of 1.4 g/dL, free light chains of 4.43 mg/dL, lactate dehydrogenases of 186 U/L, B2-microglobulin of 3.8mcg/mL, and albumin of 3.5g/dL. A bone marrow biopsy found 43% plasma cells and a peripheral blood smear showed rouleaux formation.
Case description.

Prior to the tweet chat, we shared a Twitter poll asking followers for input on what frontline regimen they would administer this patient. In total, 16 votes were cased. Lenalidomide plus low- dose dexamethasone (Rd) received 12.5% of the vote. Over half, 68.8%, voted for bortezomib (Velcade), lenalidomide (Revlimid), and dexamethasone (VRd). Carfilzomib (Kyprolis), lenalidomide, and dexamethasone (KRd) received 6.3% of the vote. Cyclophosphamide-Bortezomib-Dexamethasone (CyBorD) received 12.5% of the vote.
Poll
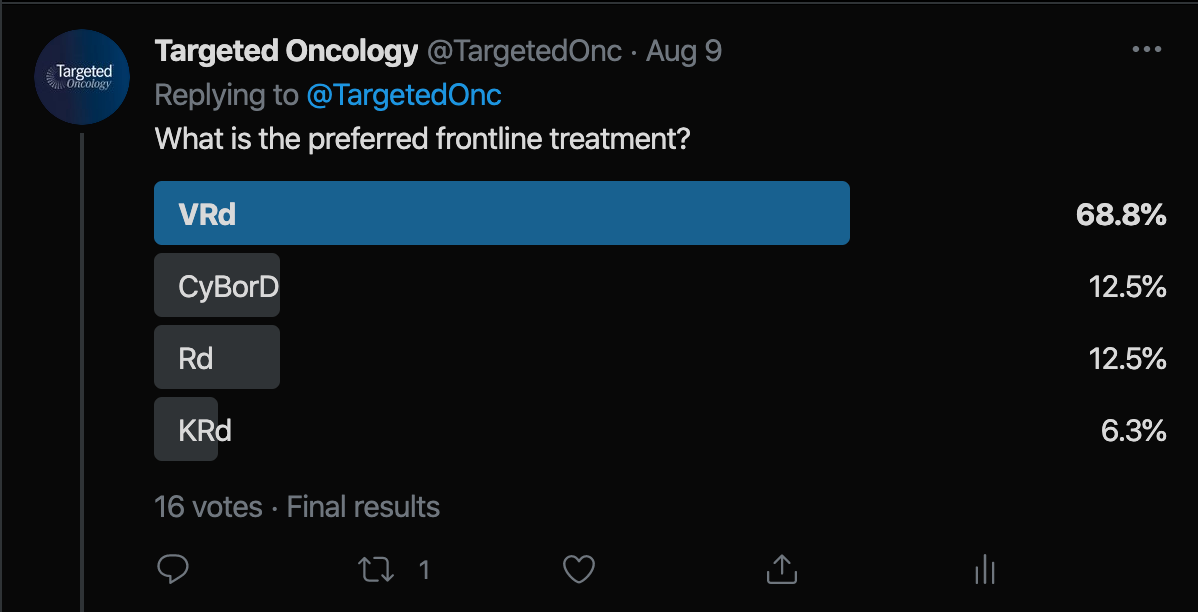
During the tweet chat, Lesokhin highlighted KRd as his preferred first-line treatment approach for transplant-ineligible patients.
“VRd is a standard regimen that is very commonly used in the community,” Lesokhin said in response to his choice. He went on to highlight a study being conducted by Memorial Sloan Kettering that found while the 2 agents had similar efficacy rates, KRd was found to be less toxic.
“The one thing that's clear is that a larger proportion of patients stopped the VRd treatment because of toxicity. And 8% had grade 3 or higher peripheral neuropathy, a higher percentage had lower grade peripheral neuropathy. And I think these are major quality of life issues that are long lasting in our patients that they need to be managed for their lifetime,” Lesokhin said.
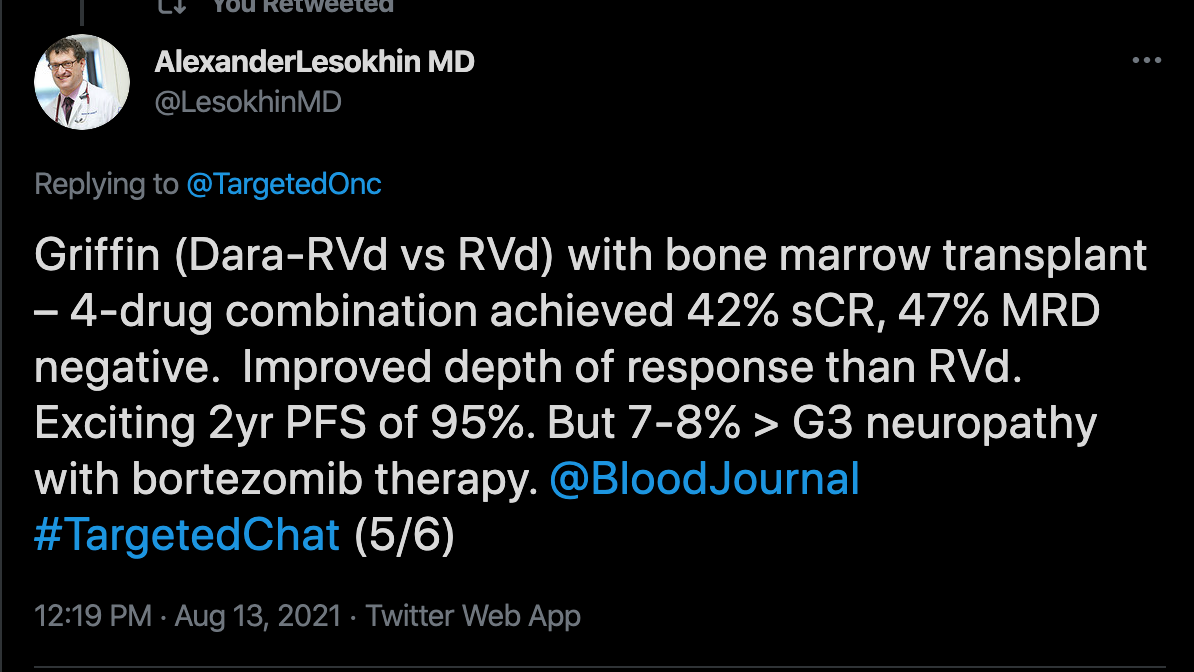
Lesokhin discusses 3 studies that support the use of KRd as a frontline treatment for newly- diagnosed multiple myeloma. The first is on the tailored treatment to MRD response (NCT02937571) which evaluated the efficacy of high dose carfilzomib. The second is the GRIFFIN Trial (NCT02874742), which compared D-RVd to RVd in newly diagnosed multiple myeloma. The third was the MANHATTAN Nonrandomized Clinical Trial (MANHATTAN trial), which looked at the safety and efficacy of weekly carfilzomib, lenalidomide, dexamethasone, and daratumumab for the treatment of newly diagnosed multiple myeloma.
Tailored Treatment to MRD Responses
“A phase 1/2 study for newly diagnosed multiple myeloma patients using high dose twice-weekly carfilzomib (45 and 56 mg/m2) in combination with lenalidomide and dexamethasone,” found that for patients who received 56mg/m2 of KRd had improved overall survival [OS] while maintaining toxicity similar to lower KRd doses.1

Median OS was not reached for the 56mg cohort. For this cohort, the 36-month progression-free survival rate was 73%. For high-risk patients, the 36-month PFS was 84% (95% CI: 66%-100%) standard-risk vs 50% (95% CI: 22%-100%) high-risk in KRD-56 cohort (P = .07) and 87% (95% CI: 72%-100%) standard-risk vs 50% (95% CI: 22%-100%) high-risk in combined cohorts (P = .03). The median number of clues it took for patients to reach MRD-negative status was 8 (range, 2-12). This is higher than the usual 4 cycles threshold.
KRd-56 was found to have a similar safety profile compared to 36mg/m2 and 45 mg/m2. All-grade cardiac treatment-related adverse events (TRAE) was 17% with a grade 3/4 rate of 3% to 6%.
The trial enrolled 37 patients and a primary end point of the number of patients with dose-limiting toxicities. In order to participate, patients must have confirmed multiple myeloma, adequate hepatic function, and have an ECOG score of 0-2. Patients with plasma cell leukemia or POEMs syndrome were not able to participate.2
The GRIFFIN Trial
The phase 2 GRIFFIN Trial randomized patients 1:1 to receive either D-RVd or RVd. Patients received 4 21-day induction cycles and 2 21-day cycles of oral lenalidomide (25 mg daily on days 1-14), subcutaneous bortezomib (1.3 mg/m2 on days 1, 4, 8, and 11), and oral dexamethasone (20 mg on days 1, 2, 8, 9, 15, and 16). Those in the D-RVd group received IV daratumumab (16 mg/kg) on days 1, 8, and 15 of cycles 1 through 4 and day 1 of post-ASCT consolidation cycles (cycles 5 and 6). Patients in this group also received IV daratumumab (16 mg/kg) on day 1 every 8 weeks or every 4 weeks. At cycle 7, all patients received maintenance therapy of oral lenalidomide (10 mg daily on days 1-21; increasing to 15 mg after 3 cycles, if tolerated).3
The study had an actual enrollment of 224 participants and a primary end point of percentage of patients with a stringent complete response. Secondary end points include overall complete response, overall response rate, percentage of patients with very good partial response, the percentage of patients with negative minimal residual disease, and duration of responses. 4
For the D-RVd cohort, the median age was 59 and was 61 in the RVd cohort. Over half of the population in each cohort was male. ECOG score, ISS disease stage, baseline creatinine clearance, and other makers were similar between both cohorts. Ninety-four percent of patients in the D-RVd group completed enrollment compared to 91.3% in the RVd group.
At the last follow-up at a median of 22.1 months, the median duration of treatment was 21.9 months in the D-RVd group and 19.5 months in the RVd group. The ORR in the D-RVd group was 99% compared to 91.8% in the RVd cohort. Greater than complete responses were seen in 51.5% of the D-RVd cohort compared to 42.3% in the RVd group. Very good partial responses were seen in 39.4% of the D-RVd cohort compared to 30.9% in the RVd cohort.
MANHATTAN Trial
The MANHATTAN trial looked at the safety and effectiveness of carfilzomib, lenalidomide, dexamethasone, and daratumumab for newly- diagnosed multiple myeloma. The study had 41 evaluable patients with a median age of 59 years. Over half, 61%, were female and 49% had high-risk multiple myeloma.5
The primary end point of MRD negativity in the bone marrow was achieved in 29 of 41 patients. The median time to MRD was 6 cycles. The rate of very good partial response or complete response was 41%. At a median follow-up of 11 months, the 1-year PFS rate was 98% and the OS was 98%. The most common grade 3 or 4 AE was neutropenia (27%), rash (9%), and lung infection (7%).

Considerations for Choosing a Regimen and Maintenance
According to Lesokhin, several factors should be taken into account when selecting a regimen for patients such as cardiac function and overall performance status. These are similar parameters to what is taken into consideration for transplant eligibility.
“I think the overarching aim of therapy is to reduce disease burden and achieve a minimal residual disease negative response and to improve quality of life without affecting long term toxicity,” Lesokhin said. “And I think the overarching sense that I have from both my own experience, as well as from the clinical data that has been reported, is that carfilzomib lenalidomide, and dexamethasone is a very good regimen that all of these ends at a high frequency.”
When it comes to maintenance, the standard of care at Memorial Sloan Kettering Cancer Center is to use lenalidomide at 10mg for 21 of 28 days until progression for patients with standard disease risk. According to Lesokhin, many patients are able to maintain MRD negativity and have a durable progression-free survival. For those with high risk disease, other medications such as a proteasome inhibitor may be added to lenalidomide.
Additionally, in the last few years, new develops are being made in myeloma space. New treatments and regimens are changing the treatment landscape regularly.
“There have been almost 10 therapies approved in the last five, six years for myeloma,” Lesokhin said. “So, it's obviously a very fast moving and very exciting space for our patients.”
At the moment, Lesokhin would recommend patients to the ADVANCE trial (NCT04268498), which looks at the safety and efficacy of daratumumab, carfilzomib, lenalidomide, and dexamethasone in newly diagnosed multiple myeloma patients. The trial has an estimated enrollment of 462 patients and a primary end point of rate of MRD. Secondary end points include overall survival, PFS, event-free survival, and rate of response.6
REFERENCES:
1.Korde N, Mastey D, Tavitian E, et al. Tailored treatment to MRD response: A phase I/II study for newly diagnosed multiple myeloma patients using high dose twice-weekly carfilzomib (45 and 56 mg/m2) in combination with lenalidomide and dexamethasone. Am J Hematol, 2021;96: E193-E196. doi: 10.1002/ajh.26150
2. High dose carfilzomib for newly diagnosed myeloma. ClinicalTrials.Gov. Accessed August 16, 2021. https://bit.ly/3m70g1M.
3. Voorhees P, Kaufman J, Laubach J Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood 2020;136 (8):936–945. doi: 10.1182/blood.2020005288
4.Study comparing daratumumab, lenalidomide, bortezomib, and dexamethasone (D-RVd) versus lenalidomide, bortezomib, and dexamethasone (RVd) in subjects with newly diagnosed multiple myeloma. ClinialTrials.Gov. Accessed August 16, 2021. https://bit.ly/3xMm3OE.
5. Landgren O, Hultcrantz M, Diamond B, et al. Safety and Effectiveness of Weekly Carfilzomib, Lenalidomide, Dexamethasone, and Daratumumab Combination Therapy for Patients With Newly Diagnosed Multiple Myeloma: The MANHATTAN Nonrandomized Clinical Trial. JAMA Oncol. 2021;7(6):862–868. doi:10.1001/jamaoncol.2021.0611
6. A Study of daratumumab, carfilzomib, lenalidomide, and dexamethasone in patients with newly-diagnosed multiple myeloma (ADVANCE). ClinicalTrials.Gov. Accessed August 16, 2021. https://bit.ly/2W1NjeD.
Gasparetto Explains Rationale for Quadruplet Front Line in Transplant-Ineligible Myeloma
February 22nd 2025In a Community Case Forum in partnership with the North Carolina Oncology Association, Cristina Gasparetto, MD, discussed the CEPHEUS, IMROZ, and BENEFIT trials of treatment for transplant-ineligible newly diagnosed multiple myeloma.
Read More