Tropomyosin-Related Kinases (TRK) Making Headway in Head and Neck Cancer
Treatment of certain head and neck cancers may soon be fundamentally transformed through the use of molecular diagnostics to dissect essential oncogenic driver pathways, and ultimately to identify targeted therapies that specifically inhibit them.
Treatment of certain head and neck cancers may soon be fundamentally transformed through the use of molecular diagnostics to dissect essential oncogenic driver pathways, and ultimately to identify targeted therapies that specifically inhibit them. Basket studies, which target genetic mutations regardless of the cancer’s site of origin, are helping to make precision medicine in oncology more attainable, even for some of the rarest forms of cancer. In particular, gene rearrangements in the tropomyosin-related kinases (TRK), a family of neurotrophin receptor tyrosine kinases encoded by theNTRK1, NTRK2,andNTRK3genes, are being increasingly recognized in head and neck cancers, and the path has already been set for targeted therapy with newly developed Trk inhibitors (Table 1). A new phase II global trial of entrectinib, a potentTrk inhibitor, will add additional clinical interest beyond typicalhead and neck cancer clinical trialsin these genes.
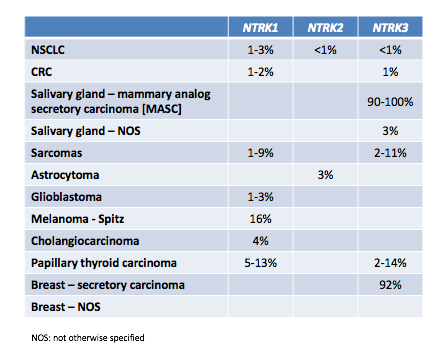
NTRKin HNSCC
Head and neck squamous cell cancers (HNSCC) are well recognized as a particularly challenging class of tumors to treat, with a sizable proportion of patients developing locoregional recurrences and metastatic disease.1,2Tropomyosin-related kinase B (TrkB), which serves as a receptor for brain-derived neurotrophic factor (BDNF) and for neurotrophic factor 4 (NT4), has been found to be a potentially important mediator of the invasive properties of HNSCC and a mediator of the epithelial-mesenchymal transition (EMT). In particular, TrkB and BDNF are expressed in >50% of HNSCC tumors, and stimulation with increases the migratory and invasive properties of HNSCC cell lines in vitro, whereas suppression of TrkB inhibits these BDNF-mediated effects. In addition, overexpression of TrkB was associated with the EMT phenotype, with E-cadherin downregulation, and Twist upregulation.1Collectively, data such as these suggest that targeting of TrkB may help prevent the metastatic and/or invasive capacity of HNSCC.
There is also evidence that TrkB targeting may help to ameliorate resistance to well-established chemotherapeutic agents in HNSCC, such as high-dose cisplatin. In one study, TrkB was identified in approximately 30% of HNSCC tumor samples, while it was absent in normal mucosal tissue. A small molecule inhibitor of TrkB, AZ64, displayed antiproliferative effects on HNSCC cell lines in this study and abrogated tumor migratory capacity in vitro.2In addition, genetic inhibition of TrkB, as well as inhibition with AZ64, was found to augment the antiproliferative activity of cisplatin, and cells resistant to cisplatin could be resensitized to cytotoxic therapy with cisplatin with the use of AZ64.2Data from this study thus suggested that oncogenic signaling by TrkB may be at least one important mediator of cisplatin resistance in HNSCC, which could be overcome with the use of small molecule TRK inhibitors.2Subsequent experiments in this regard have begun to dissect the molecular pathways activated by the BDNF-TrkB axis that may be important in suppressing apoptosis induced by cisplatin, leading to chemotherapy resistance.3
Trk in Thyroid Cancers
Gene rearrangements of theNTRKfamily and other genes have been well-recognized in thyroid tumors, particularly papillary thyroid cancer (PTC).4,5For example, rearrangements ofNTRK1have been found in both spontaneously-arising and radiation-associated PTCs (in one study, 12% and 14%, respectively), and activating rearrangements of this gene have been implicated in a subset of radiation-associated PTC, but not in adenomas.5
In 1999, changes inNTRKfamily gene expression were noted in patients with medullary thyroid cancer (MTC), a cancer arising from the thyroid C cell.6Researchers found that a subset of normal C cells expressed TrkB, but lacked expression of TrkA or TrkC, and in patients with C-cell hyperplasia they found consistent TrkB staining by immunohistochemistry. By comparison, with progression from C-cell hyperplasia to MTC, marked changes were seen in Trk expression, with staining for TrkB much less prominently observed in late-stage MTCs, strong TrkC staining observed in 87% of MTC tumors, and strong-to-moderate expression of TrkA in 68% of MTC tumors.6Moreover, patients with the most aggressive and metastatic tumors leading to death were characterized by strong TrkC staining and were negative for TrkB.
Additional findings from this study showed that expression of the different Trk receptors in cultured MTC cells was associated with an increase in ligand-independent growth (in the case of TrkC and TrkA) and enhanced ligand-dependent growth (in the case of TrkB). TrkC- and TrkA-expressing cells also displayed increased clonogenicity and tumorigenicity in nude mice, in contrast to TrkB-expressing MTC cells, which were markedly inhibited in this capacity; the latter effect appeared to be due to suppression of angiogenic capacity via TrkB-mediated suppression of vascular endothelial growth factor (VEGF).6
A chromosome 1NTRKrearrangement calledNTRK1-T1has been identified in PTC, and consists of the carboxy terminal portion ofNTRK1(including the tyrosine kinase domain) and the amino terminal portion of the translocated promoter region (TPR) gene.7,8In elegantly designed transgenic mouse experiments, theNTRK1-T1gene rearrangement has been shown to be oncogenic and induce transformation of thyroid epithelium. When expressed in a thyroid-specific manner, the TrkA-T1 fusion product induced thyroid hyperplasia and carcinoma, with 54% of the transgenic thyroids displaying histologically deranged thyroid architecture, which was absent in their nontransgenic littermates.8A thyroid carcinoma morphologically resembling human thyroid cancer was also observed in 78% of transgenic mice expressing TrkA-T1 by 7 months of age. Collectively, these experimental findings demonstrated the oncogenic potential of this particularNTRKrearrangement in thyroid carcinoma.8Subsequent cytogenetic analyses of these TrkA-T1-positive thyroid tumors have also begun to identify potential downstream candidate genes that may be important in thyroid tumorigenesis.9
NTRKin Salivary Cancers
Conventional pharmacologic treatments are largely ineffective for cancers of the salivary glands, including adenoid cystic carcinoma (ACC), the most common histotype, and salivary duct carcinoma (SDC), the most aggressive type of salivary cancer.10As such, targeted therapies would offer an attractive treatment option for salivary cancers, once the molecular drivers have been sufficiently elucidated. Earlier studies have suggested a role for Trks and neurotrophins in the normal physiology of salivary epithelial glands; however, salivary cancers are rare and data on Trk expression in these tumors are limited.11Up to 90% of MASC salivary cancers are positive for this NTRK rearrangement, and it is considered to be a driver and targetable mutation.
In 2008, Negri and coworkers assessed the expression of TrkA and other tyrosine kinase receptors in a series of SDCs (n = 9), ACCs (n = 12), and normal salivary glands (n = 8).10They found TrkA to be expressed in all normal samples, whereas it was overexpressed in 6 of 9 SDCs and 3 of 12 ACCs; its ligand nerve growth factor (NGF) also appeared to be coexpressed in all cases, and there was evidence for autocrine/paracrine loop activation in both SDC and ACC.10In a Chinese study of 47 patients with ACC, expression of TrkA and VEGF receptor 2 (VEGFR2) was detected in 87% and 85% of samples, respectively, by immunohistochemical means; moreover, perineural invasion and recurrence were higher in the patients that expressed high levels of these receptors.12Ivanov et al have also implicated a role for TrkC and NT-3 in ACC; TrkC was highly expressed in 17 of 18 ACCs examined, but not in mucoepidermoid salivary carcinomas or in HNSCCs, and evidence of an autocrine NT-3/TrkC-signaling loop was suggested.13Despite these results, however, a definitive correlation betweenNTRKexpression and prognosis/progression in ACC remains to be rigorously established.
Entrectinib Clinical Response in a Salivary Gland Patient
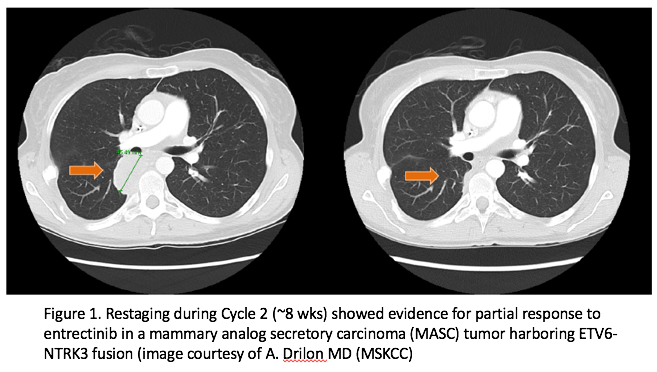
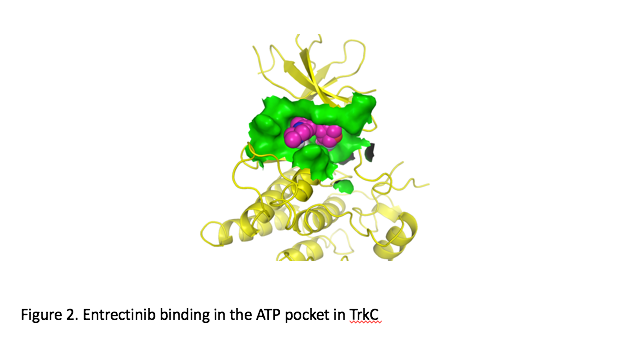
Results from two Phase 1 trials of entrectinib, a promising kinase inhibitor of Trk, ROS1 and ALK fusions were presented at this year’s European Society for Medical Oncology (ESMO) meeting. As part of this experience, a 42-year-old female with salivary gland carcinoma of the parotid was treated. The patient had multiple prior therapies, including surgery/EBRT, vinorelbine, carboplatin/paclitaxel, doxorubicin, and crizotinib. Further evaluation at Memorial Sloan Kettering Cancer Center revealed that the patient’s tumor harbored anETV6-NTRK3gene rearrangement, making the proper classification of her tumor to be mammary analog secretory carcinoma (MASC). She was subsequently enrolled in Ignyta’s STARTRK-1 trial at in December of 2014. The patient had an ECOG performance status of 1 and baseline staging showed multiple paraspinal and pleural masses. After receiving entrectinib for approximately eight weeks, restaging studies showed evidence of a partial response (Figure 1 and 2).15Continued treatment resulted in additional tumor shrinkage with a maximal tumor reduction of 89%. The patient remained progression-free for seven months but remained on therapy because of evidence of continued clinical benefit.
A Growing Need for Molecular Profiling
Alan L. Ho, MD, PhD, a medical oncologist at the Geoffrey Beene Cancer Research Center at Memorial Sloan Kettering Cancer Center, specializes in the treatment of head and neck cancers. Ho states that the current evidence showsNTRKmutations to be comparatively rare in these malignancies. “Genomic profiling efforts to date for head and neck cancers, and to a much lesser extent for salivary cancers, suggest that changes in theNTRKgene family are likely rare molecular events for these diseases. Still, detection ofNTRK rearrangementscan be incredibly important for patients whose tumors harbor them. For instance, the recent identification of theETV6-NTRK3rearrangement has helped define a new pathologic subset of salivary cancers, known as mammary analogue secretory carcinoma (MASC). Identifying such groups enriched forNTRKrearrangements now also takes on potential therapeutic significance, given the availability of Trk-targeted, experimental therapies,” Ho said.
Ho also noted that genomic profiling is, at present, largely uncommon in head and neck cancers. “Molecular testing is not standard for patients with head/neck and salivary cancers. However, with the increasing availability of experimental and approved agents that are designed to inhibit genetically altered targets, we are just beginning to touch on the potential of molecular testing to identify rational therapeutic options for our patients, particularly those with rare diseases for whom standard treatment options do not exist and greater clinical guidance is most needed,” Ho stated. In addition, notwithstanding the above data onNTRKin salivary and other cancers, Ho proposed that additional data are needed to determine the exact role of these molecular alterations. “While profiling efforts suggest that changes in theNTRKfamily of genes may occur, more studies are needed to better elucidate the biologic impact these changes may have on the oncogenesis and overall oncogenic phenotype of these cancers," he said.
TargetingNTRK: The Entrectinib Basket Study
The recognition of alteredNTRKexpression, of molecular alterations inNTRKgenes, and of the potential role ofNTRKin HNSCC, thyroid, salivary, and indeed many other types of cancer, is not new. In the age of molecular diagnostics, and with the growing availability of therapies specifically targeted toNTRK, however, there is likely to be an increased interest in finding patients who haveNTRKmutations and rearrangements, particularly in those rare cancers that are most lacking in effective therapies. A late-breaking presentation at this year’s ESMO meeting by Siena et al updated the results of two phase I trials with entrectinib, a pan-Trk, ROS1, and ALK inhibitor in solid tumors (total 92 patients enrolled). An Overall Response Rate of 72% (13/18 patients) and a disease control rate of 89% (16/18 patients) was observed in the population withNTRK,ROS1orALKgene rearrangements. Of special interest was a nonsmall cell lung cancer patient withNTRK1rearrangement who had a partial response along with complete resolution of numerous CNS metastatic lesions. The results have prompted the initiation of a global, phase II basket study (STARTRK-2) that will be designed to identify patients with these molecular alterations and further investigate the activity of entrectinib across multiple tumor types.15
“STARTRK-2 and other trials like it represent exciting, new treatment opportunities for patients with tumors that harbor the appropriate genetic aberrations,” said Dr. Ho. “The challenge today is being able to more broadly apply molecular profiling to more efficiently identify the appropriate patients for these trials. While the incidence of these alterations may be low, identifying these changes may be an important way to make available new experimental, potentially effective therapeutic options to patients.”
For more information on the phase II trial for entrectinib in patients withNTRK,ROS1, andALKgene rearrangements, please visitwww.STARTRKtrials.com.
References
- Kupferman ME, Jiffar T, El-Naggar A, et al. TrkB induces EMT and has a key role in invasion of head and neck squamous cell carcinoma.Oncogene.2010;29(14):2047-2059.
- Yilmaz T, Jiffar T, de la Garza G, et al. Theraputic targeting of Trk suppresses tumor proliferation and enhances cisplatin activity in HNSCC.Cancer Biol Ther.2010;10(6):644-653.
- Lee J, Jiffar T, Kupferman ME. A novel role for BDNF-TrkB in the regulation of chemotherapy resistance in head and neck squamous cell carcinoma.PLoS One.2012;7(1):e30246.
- Greco A, Borrello MG, Miranda C, Degl’Innocenti D, Pierotti MA. Molecular pathology of differentiated thyroid cancer.Q J Nucl Med Mol Imaging.2009;53(5):440-453.
- Bounacer A, Schlumberger M, Wicker R, et al. Search for NTRK1 proto-oncogene rearrangements in human thyroid tumours originated after therapeutic radiation.Br J Cancer.2000;82(2):308-314.










2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.

