Treatment of Smoldering Myeloma Delays Disease Progression, Yet Questions Remain
There are many greatdebates in the field of multiple myeloma, and one that is becoming increasingly relevant in the era of modern therapies is whether or not to treat patients with asymptomatic disease. While the etiology of MM remains unknown, a major advancement in understanding myeloma pathogenesis has been the observation that all patientsprogress, albeit at differing rates, through an asymptomatic phase of either monoclonal gammopathy of undetermined significance or smoldering MM.
Marc Braunstein, MD, PhD

Marc Braunstein, MD, PhD
There are many great debates in the field of multiple myeloma (MM), and one that is becoming increasingly relevant in the era of modern therapies is whether or not to treat patients with asymptomatic disease. While the etiology of MM remains unknown, a major advancement in understanding myeloma pathogenesis has been the observation that all patients progress, albeit at differing rates, through an asymptomatic phase of either monoclonal gammopathy of undetermined significance (MGUS) or smoldering MM.1,2Both of these precursor conditions lack the end organ damage that defines symptomatic MM.2
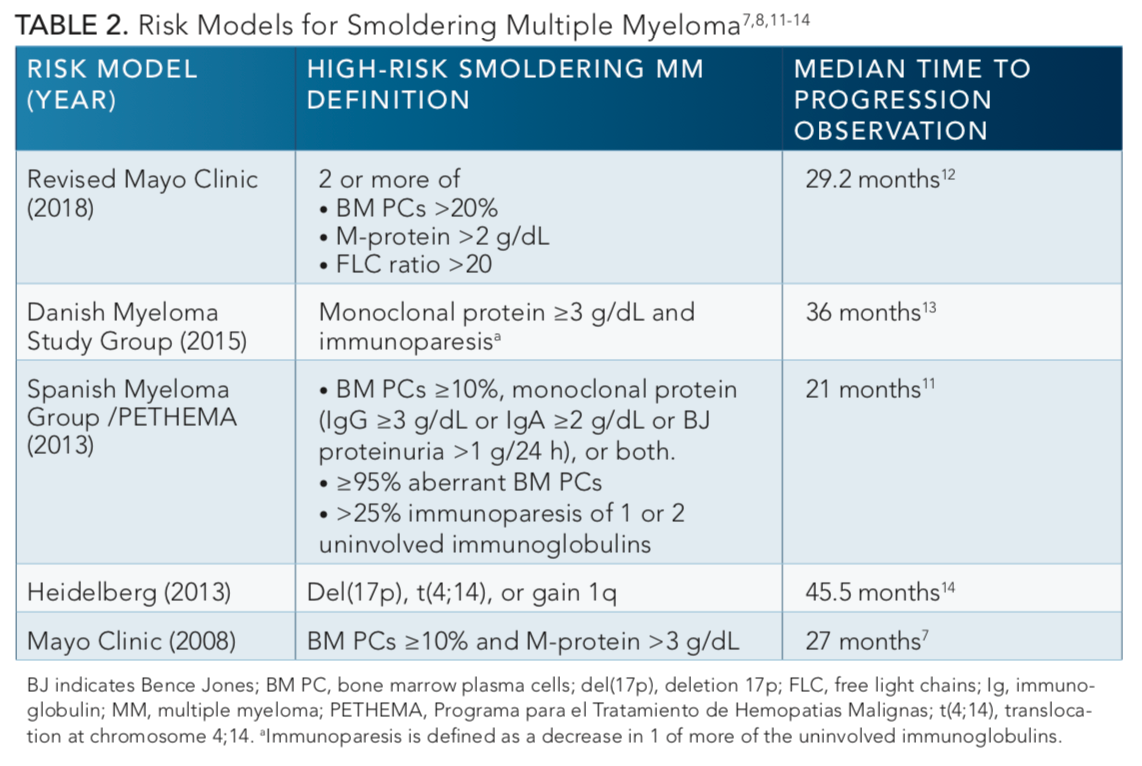
Several factors have been suggested to account for differences in the time to progression (TTP) among patients with asymptomatic MM, such as immunoglobulin isotype, serum-free light chains, and cytogenetics.3,4Nearly 5% of the population over age 70 are living with MGUS, and because the expected rate of progression to symptomatic MM is 1% per year in immunoglobulin M MGUS, the convention remains to monitor these individuals for signs of progression.1,5Despite at least a 10% greater risk for progression per year within the first 5 years of diagnosis versus MGUS, patients with smoldering MM historically have also been observed, in part because of the previous lack of stratifying risk factors and also a concern for undue toxicities associated with available therapies at the time.6,7
Patients with smoldering MM represent a heterogeneous population, with a subgroup of about one-third of patients considered to be high-risk for progression within 2 years.8In fact, in 2014 the International Myeloma Working Group (IMWG) revised the definition of symptomatic myeloma to include patients who would have previously been considered to have “ultrahigh risk” smoldering MM with 60% or more clonal plasma cells in the bone marrow, an involved to uninvolved serum-free light chain ratio of ≥100, or >1 focal bone lesion on MRI.9Thus, it is important to define whether patients are truly asymptomatic and lack myeloma defining events (TABLE 19) and to pay particular attention to higher resolution imaging to assess for lytic bone disease.10
Evidence Supporting Treatmentof Smoldering MM
As the available agents to treat symptomatic MM have increased over the past decade coincident with improved safety profiles, there has been an increasing focus on studies intervening with earlier treatment at the smoldering phase. However, there still remains an absence of a standard approach for initiating treatment for smoldering MM, partly because studies prior to 2014 included patients with ultrahigh risk who would now be considered to have symptomatic disease. In addition, the definition of high-risk smoldering MM continues to evolve (TABLE 2).7,8,11-14Earlier studies combining melphalan and prednisone in patients with asymptomatic disease demonstrated a measurable reduction in paraprotein levels and delay in progression, but no effect on response rate or survival.15As shown inTABLE 3,13,16,17more recent data from randomized controlled studies using novel anti-myeloma agents provide additional support to the impact of treatment on delaying progression, and importantly, these studies also demonstrate survival benefits versus observation alone.
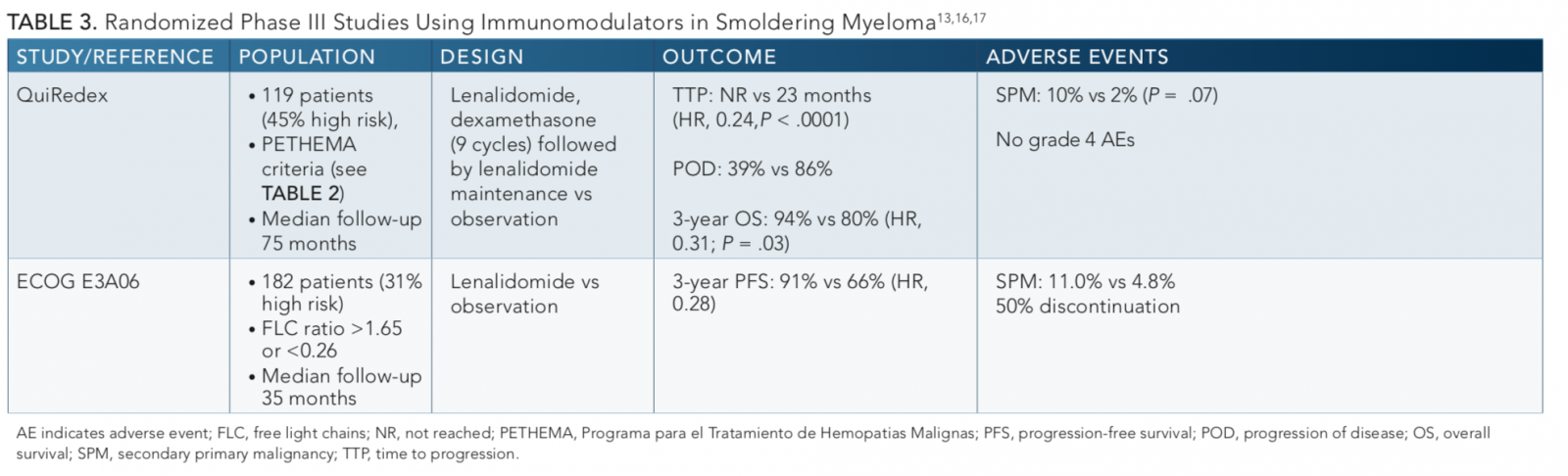
The largest study to date by the Spanish Myeloma Group PETHEMA (QuiRedex) led by Maria Mateos, PhD, randomized 119 patients with smoldering MM to either lenalidomide (Revlimid) plus dexamethasone for 9 cycles followed by lenalidomide maintenance versus observation with a longer-term follow-up11,16published in 2016. The primary end point of this phase III study was TTP. At a median follow-up of 75 months, the median TTP was not reached in the treatment arm compared with 23 months in the control arm (P<.0001). Progression to symptomatic MM occurred in 39% in the experimental arm versus 86% in the control arm. Although the median overall survival (OS) was not reached in either arm, there was a 57% reduction in the risk of death in the experimental arm (HR, 0.43;P= .024). However, there was no difference in survival in patients who received subsequent treatments at the time of progression to symptomatic disease and no differences in response rate to subsequent therapy. The major grade 3 adverse events (AEs) with treatment were infection, asthenia, and neutropenia, but the overall rate for each was ≤6%. While secondary primary malignancies were more common in the treatment arm (10% vs 2%), the differences were not significant (P= .07). However, criticism of this study included that the ultrahigh risk patients were included, more patients discontinued therapy in the treatment arm, and patients were not screened for lytic lesions with advanced imaging techniques. Nevertheless, this study provided compelling evidence for use of an immunomodulator in smoldering MM.
A study from ECOG-ACRIN (E3A06) presented at the 2019 American Society of Clinical Oncology Annual Meeting by Sagar Lonial, MD,17and later published in theJournal of Clinical Oncology18randomized 182 patients with smoldering MM to either single-agent lenalidomide or observation. MRI imaging was required to rule out lytic bone disease. The primary endpoint was progression-free survival (PFS). At a median follow-up of 35 months, the 3-year PFS rate was 91% with lenalidomide versus 66% in the observation arm (HR, 0.28,P= .0005). Fatigue was the most common grade ≥3 AE. The rate of second primary malignancies at 3 years was 11.0% versus 4.8% in the observation arm. Criticisms of the trial included that the treatment discontinuation rate was 59% (due to AEs or withdrawal) and that much of the PFS benefit of lenalidomide was derived from the 25 high-risk patients, who were a small portion of the overall population.
Taken together, these studies clearly show a benefit of lenalidomide in delaying progression to MM. Application of these data in practice will require careful considerations of individual patient characteristics, costs, and risk versus benefits, in a similar fashion to our decision-making process for symptomatic MM.19
Current and Future Directions
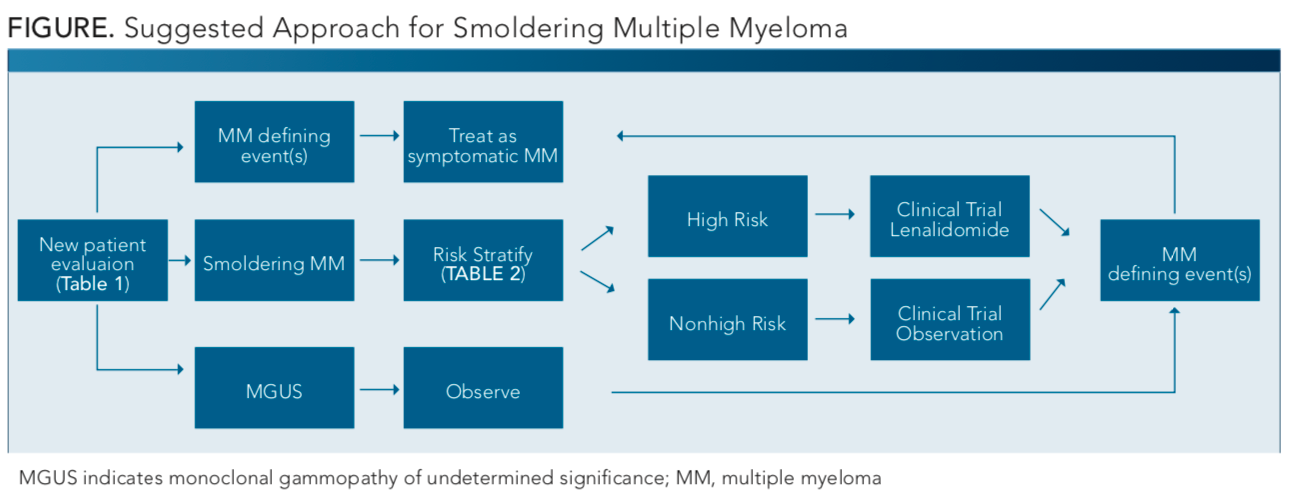
At the present time, a suggested approach toward diagnosis of smoldering myeloma, as shown in theFIGURE, based on the recent randomized studies can help guide the management of smoldering MM, though this is not without limitations. Treatment of patients with smoldering MM is still not established in routine clinical practice; therefore, more studies are required to help guide timing and approach. Clinical trials are preferred, because lenalidomide alone or in combination with dexamethasone can delay but not entirely prevent progression to symptomatic MM.
As shown inTABLE 4, there are several ongoing investigations aimed at deepening the response to novel agents and their combinations. For example, in the phase II GEM-CESAR study, an aggressive approach is used to treat high-risk smoldering MM, similar to that used with symptomatic patientswith induction therapy of carfilzomib (Kyprolis), lenalidomide, and dexamethasone (KRd); consolidation with autologous stem cell transplant followed by KRd for 2 cycles; and Rd maintenance—showed a progressive improvement in response, including rates of measurable residual disease (MRD) negativity.20 In this study of 90 patients with smoldering MM (including ultrahigh risk disease) <70 years, the MRD-negativity rate increased from 30% after induction to 56% and 61% after transplant and consolidation, respectively, along with an impressive 100% objective response rate at the end of consolidation. Most of the studies shown inTABLE 4used PFS as a surrogate end point because OS takes many more years to assess, and therefore we must pay close attention to any emerging long-term AEs in patients who would otherwise have been under observation alone. In addition, these ongoing trials of smoldering MM use various criteria to define high-risk disease, necessitating caution when comparing results between studies.
Given that patients with ultrahigh risk smoldering MM are now considered to have symptomatic disease, smoldering MM is becoming an increasingly smaller percentage of plasma cell dyscrasias, making it more challenging to identify and study patients who are truly asymptomatic. Eventually, we may even consider patients with high-risk smoldering MM as having symptomatic disease, as was done with ultra–high risk smoldering MM with the 2014 revised IMWG criteria.9The ongoing Dana-Farber PROMISE study (NCT03689595) and The University of Texas MD Anderson Cancer Center study (NCT02726750) are performing prospective genomic evaluations of a large cohort of asymptomatic patients and these may shed light on additional biological risk factors for development of symptomatic myeloma. Again, we must stress the importance of enrolling patients with smoldering myeloma in registry or interventional studies in order to guide future interventions. Whether we can truly extinguish myeloma at the smoldering phase remains to be determined. Until then, the debate continues on how best to approach these patients.
References
- Kyle RA, Larson DR, Therneau TM, et al. Long-term follow-up of monoclonal gammopathy of undetermined significance. N Engl J Med. 2018;378(3):241-249, doi: 10.1056/NEJMoa1709974.
- Morgan GJ, Walker BA, Davies FE, et al. The genetic architecture of multiple myeloma.Nat Rev Cancer.2012;12(5):335-348 doi: 10.1038/nrc3257.
- Landgren O, Hofmann JN, McShane CM, et al. Association of immune marker changes with progression of monoclonal gammopathy of undetermined significance to multiple myeloma.JAMA Oncol. 2019;5(9):1293-1301. doi: 10.1001/jamaoncol.2019.1568 (2019).
- Rajkumar SV, Landgren O, Mateos MV, et al. Smoldering multiple myeloma.Blood. 2015;125(20):3069-3075. doi: 10.1182/blood-2014-09-568899.
- Go RS, Rajkumar SV. How I manage monoclonal gammopathy of undetermined significance.Blood.2018;131(2):163-173, doi: 10.1182/blood-2017-09-807560.
- Ghobrial IM, Landgren O. How I treat smoldering multiple myeloma.Blood. 2014:124(23):3380-3388 doi: 10.1182/blood-2014-08-551549.
- Kyle RA, Remstein ED, Therneau TM, et al. Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med. 2007;356(25):2582-2590, doi: 10.1056/NEJMoa070389.
- Cocito F, Mangiacavalli S, Valeria Ferretti V, et al. Smoldering multiple myeloma: the role of different scoring systems in identifying high-risk patients in real-life practice. Leuk Lymphoma.2019;6:1-7. doi: 10.1080/10428194.2019.1620948.
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-e548, doi: 10.1016/S1470-2045(14)70442-5.
- Hillengass J, Usmani S, Rajkumar SV, et al. International Myeloma Working Group consensus recommendations on imaging in monoclonal plasma cell disorders.Lancet Oncol. 2019;20(6):e302-e312. doi: 10.1016/S1470-2045(19)30309-2.
- Mateos MV, San Miguel JF. Treatment for high-risk smoldering myeloma.N Engl J Med. 2013;369(18):1764-1765. doi: 10.1056/NEJMc1310911.
- Lakshman A, Rajkumar SV, Buadi FK, et al. Risk stratification of smoldering multiple myeloma incorporating revised IMWG diagnostic criteria. Blood Cancer J. 2018;8(6):59. doi: 10.1038/s41408-018-0077-4.
- Sørrig R, Klausen TW, Salomo M, et al. Smoldering multiple myeloma risk factors for progression: a Danish population-based cohort study. Eur J Haematol. 2016;97(3):303-309. doi: 10.1111/ejh.12728.
- Neben K, Jauch A, Hielscher T, et al. Progression in smoldering myeloma is independently determined by the chromosomal abnormalities del(17p), t(4;14), gain 1q, hyperdiploidy, and tumor load. J Clin Oncol. 2013;31(34):4325-4332, doi: 10.1200/JCO.2012.48.4923.
- He Y, Wheatley K, Clark O, Glasmacher A, Ross H, Djulbegovic. Early versus deferred treatment for early stage multiple myeloma. Cochrane Database Syst Rev. 2003;(1):CD004023 doi: 10.1002/14651858.CD004023.
- Mateos MV, Hernández MT, Giraldo P, et al. Lenalidomide plus dexamethasone versus observation in patients with high-risk smouldering multiple myeloma (QuiRedex): long-term follow-up of a randomised, controlled, phase 3 trial. Lancet Oncol. 2016;17(8):1127-1136 doi: 10.1016/S1470-2045(16)30124-3.
- Lonial S, Jacobus SJ, Weiss M, et al. E3A06: Randomized phase III trial of lenalidomide versus observation alone in patients with asymptomatic high-risk smoldering multiple myeloma [abstract 8001]. J Clin Oncol. 2019;37(suppl 15). doi: 10.1200/JCO.2019.37.15_suppl.8001.
- Lonial S, Jacobus S, Fonseca R, et al. Randomized trial of lenalidomide versus observation in smoldering multiple myeloma [published online October 25, 2019].J Clin Oncol. doi: 10.1200/JCO.19.01740.
- Antoine-Pepeljugoski C, Braunstein MJ. Management of newly diagnosed elderly multiple myeloma patients.Curr Oncol Rep. 2019;21(7):64. doi: 10.1007/s11912-019-0804-4.
- Mateos M-V, Martínez-López J, Rodríguez-Otero P, et al. Curative strategy (GEM-CESAR) for high-risk smoldering myeloma: carfilzomib, lenalidomide and dexamethasone (KRd) as induction followed by HDT-ASCT consolidation with KRd and maintenance with Rd [abstract S871]. Presented at: 2019 European Hematology Association Congress; June 13-16, 2019; Amsterdam, The Netherlands. bit.ly/31KuN7X.




