Sotorasib, Trametinib Combo Shows Safety and Antitumor Activity in CRC and NSCLC
Results from the phase 1b CodeBreak 101 study were favorable for the combination of sotorasib and trametinib in patients with colorectal cancer and non–small cell lung cancer.
Suresh S. Ramalingam, MD, FACP, FASCO
In heavily pretreated patients with KRAS G12C–mutated solid tumors, the combination of sotorasib (Lumakras) with the MEK inhibitor trametinib was shown to be safe, with antitumor activity observed in patients with and without prior KRAS G12C inhibitor therapy, according to the phase 1b CodeBreak 101 study (NCT04185883). Findings were presented at the American Association for Cancer Research-National Cancer Institute-European Organization for Research and Treatment of Cancer Virtual International Conference on Molecular Targets and Cancer Therapeutics by lead author Suresh S. Ramalingam,MD, FACP, FASCO.1
In the previous CodeBreaK 100 study (NCT03600883), sotorasib monotherapy demonstrated an objective response rate (ORR) of 37.1%, a median progression-free survival of 6.8 months, and a median overall survival of 12.5 months in patients with non–small cell lung cancer (NSCLC). In patients with colorectal cancer (CRC), an ORR of 7.1% and a median PFS of 4.0 months were reported.2
“Because of these previous encouraging findings, we proceeded to explore novel combination therapies in CRC, NSCLC, and other solid tumors,” Ramalingam said.
Ramalingam is a professor in the Department of Hematology and Medical Oncology and the Roberto C. Goizueta Distinguished Chair for Cancer Research at Emory University School of Medicine and executive director of Winship Cancer Institute of Emory University in Atlanta, Georgia.
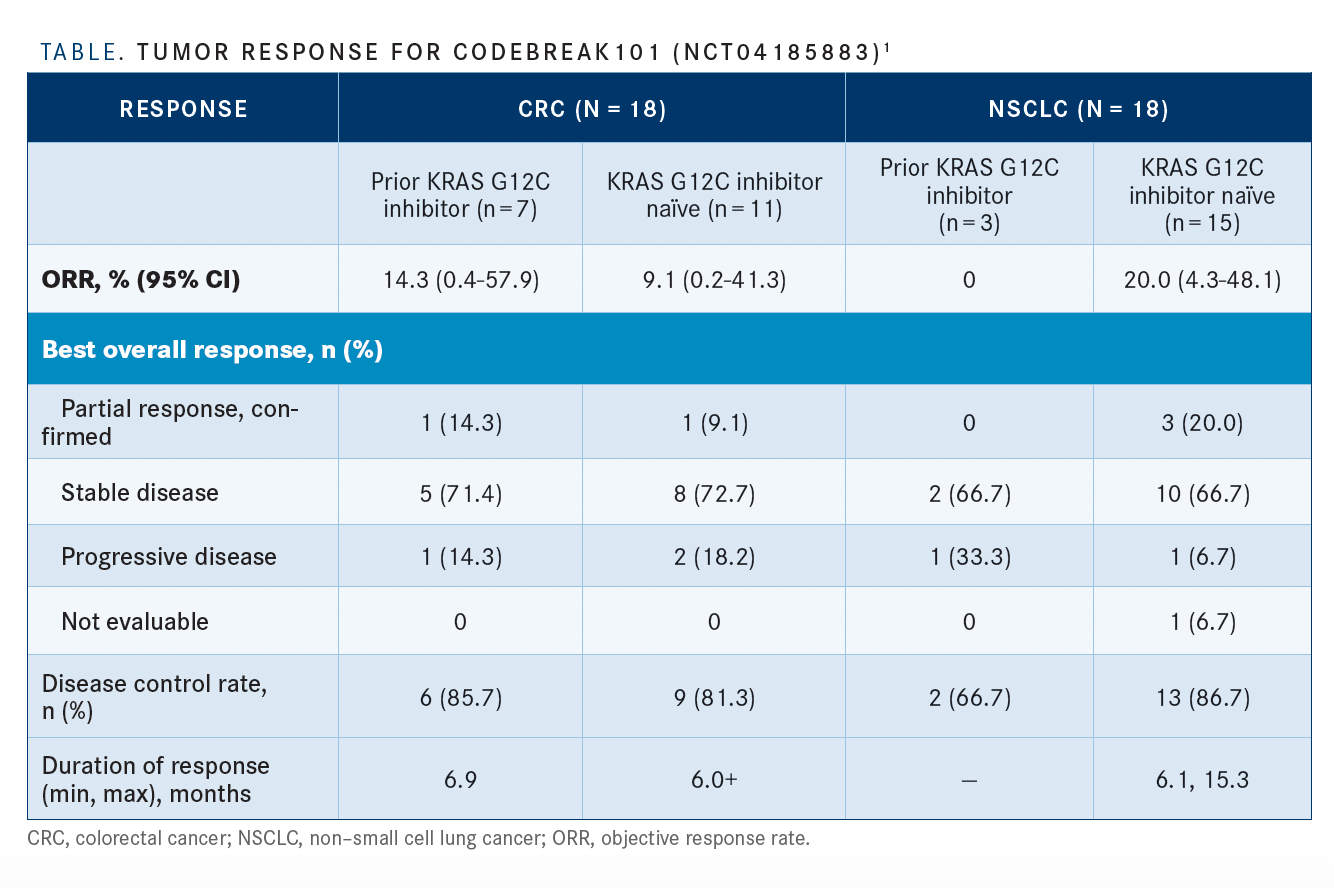
Regarding tumor response, 6 of 7 patients (86%) with CRC and 2 of 3 patients (67%) with NSCLC achieved disease control (TABLE1).
“For patients with CRC who had previously been treated with a G12C inhibitor, the objective response rate was 14.3%,” Ramalingam said. “The disease control rate was approximately 85%. The duration of response was a median of 6.9 months. For patients with CRC who were G12C inhibitor naïve, the response rate was 9.1% and the disease control rate was 82%.
The majority (67%) of patients with CRC who received sotorasib plus trametinib experienced a decrease in target lesion size. Disease control was achieved in 86% of patients with prior KRAS G12C inhibitor therapy. One patient had a deep partial response, Ramalingam said.
Investigators reported a decrease in target lesion size in 88% of patients with NSCLC who received sotorasib plus trametinib. Disease control was achieved in 2 of 3 patients (67%) who had prior KRAS G12C inhibitor therapy. Two patients had stable disease, and 1 patient had progressive disease. Seven patients had disease control for more than 6 months, and 4 patients are undergoing treatment.
CodeBreak 101 is a comprehensive, global master protocol designed to evaluate safety and efficacy of sotorasib in more than 10 treatment combinations in patients with advanced solid tumors harboring KRAS G12C mutations. A total of 41 patients were enrolled in the study: 18 patients had CRC, 18 patients had NSCLC, and 5 had other solid tumors.
During the virtual presentation, Ramalingam said that in the CRC cohort 3 patients received 960 mg sotorasib plus 1 mg trametinib and 15 patients received 960 mg sotorasib plus 2 mg trametinib.
In this cohort, 7 patients had been treated with a prior KRAS G12C inhibitor. In the NSCLC cohort, 18 patients were administered 960 mg sotorasib plus 2 mg trametinib, and 3 patients had been previously treated with a prior KRAS G12C inhibitor.
In the solid tumor cohort, 3 patients received 960 mg sotorasib plus 2 mg trametinib, and 2 patients had received prior treatment with a KRAS G12C inhibitor.
Investigators reported that the primary end points of CodeBreak 101 were dose-limiting toxicities (DLTs) and adverse events; secondary end points were efficacy and pharmacokinetics. Median treatment duration of the combination therapy was 2.8 months (range, 0.3-12.9), and no drug interactions were observed between sotorasib and trametinib.
Overall, baseline characteristics revealed that the majority of patients (55.6%) had received multiple prior treatment regimens in both the CRC and NSCLC cohorts, with a median of 3 prior lines of therapy. In the CRC cohort, the median age was 52 years (range, 34-72), the majority were male (55.6%), and 94.5% had an ECOG performance status of 0 to 1. Nearly 40% (38.9%) of patients in this cohort had been treated with a KRAS G12C inhibitor.
In the NSCLC cohort, the median age was 70 years (range, 50-84), the majority were male (55.6%), and 94.3% had an ECOG performance status of 0 to 1. Three patients (16.7%) in this cohort had been treated with a prior KRAS G12C inhibitor.
In the study, no fatal treatment-related adverse events (TRAEs) were observed, with 39 patients (95.1%) exhibiting any-grade TRAEs. TRAEs greater than grade 3 were seen in 34.1% of patients. TRAEs leading to dose modification were reported in 46.3% of patients, and 24.4% of patients discontinued treatment because of TRAEs. One patient (3.3%) reported a DLT.
Investigators reported that no new or unexpected toxicities were identified. The most common all-grade TRAEs identified were diarrhea (43.9%), rash (34.1%), dermatitis acneiform (31.7%), and nausea (29.3%).
“Ejection-fraction decrease and blood creatine phosphokinase increase were the most common grade 3 or higher TRAEs, with each occurring in 3 out of 41 patients,” Ramalingam said. CodeBreak 101 continues to evaluate the combination of sotorasib with other anticancer therapies, including targeted antibodies, small molecules, immunotherapy, and chemotherapy.
REFERENCES:
1. Ramalignam SS, Fakih M, Strickler JH, et al. A phase 1b study evaluating the safety and efficacy of sotorasib, a KRAS G12C inhibitor, in combination with trametinib, a MEK inhibitor, in KRAS p.G12C-mutated solid tumors. Presented at: American Association for Cancer Research- National Cancer Institute-European Organization for Research and Treatment of Cancer (AACR-NCI-EORTC) Virtual International Conference on Molecular Targets and Cancer Therapeutics. October 7-10, 2021.
2. Skoulidis F, Li BT, Govindan R, et al. Overall survival, and exploratory subgroup analyses from the phase 2 CodeBreaK 100 trial evaluating sotorasib in pretreated KRAS p.G12C mutated non-small cell lung cancer. J Clin Oncol. 2021;39(suppl 15):9003-9003. doi: 10.1200/JCO.2021.39.15_suppl.9003
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More