Roundtable Discussion: Panel Explains the Different Treatment Options in Nonmetastatic CRPC
Matthew Smith, MD, PhD, led a panel discussion around the various treatment options for a patient with nonmetastatic castration-resistant prostate cancer.
Matthew Smith, MD, PhD

Matthew Smith, MD, PhD, the Claire and John Bertucci Endowed chair in Genitourinary Cancers, professor of Medicine, Harvard Medical Schoolm and director, Genitourinary Malignancies Program at Massachusetts General Hospital, led a panel discussion around the various treatment options for a patient with nonmetastatic castration-resistant prostate cancer (CRPC).
Joining Smith on the panel were Terry L. Evans, MD Elaine Beed, MD, Jose Silva, MD, Jyothika Mamadgi, MD, Moses S. Raj, MD, Jason Bierenbaum, MD, Daniel Efiom-Ekaha, MD, Neeta P. Pathe, MD, and Shifeng S. Mao, MD, PhD.
CASE SUMMARY:
In October 2016, a 57-year-old Black man was referred to the urology department with a PSA (prostate-specific antigen) of 6.8 ng/mL. His medical history included seizures that were controlled with oxcarbazepine. His mother and sister had a history of breast cancer, and his brother had a history of pancreatic cancer. A multiparametric MRI scan showed a 58 cc index lesion to his left prostate zone, and prostate imaging reporting and data system showed it to be 4/5, 1.8 cm. Three months later, he had a robotically assisted radical prostatectomy and extended lymph node dissection. Six weeks post operation, the patient had a PSA of 0.15 ng/mL and baseline serum testosterone of 420 ng/mL.
Androgen depravation therapy was initiated with leuprolide depot at 45 mg. The patient returned in August 2019. His PSA doubling time (PSA-DT) was 8.6 months with a PSA of 1.2 ng/mL. In October 2019 he was restaged, and bone scans showed he was negative for metastatic disease with an ECOG performance score of 0. In October 2020, the patient’s PSA was 3.81 ng/mL.
DISCUSSION QUESTIONS
- Is there a role for hereditary germline testing in this patient?
- When do you restage/image this patient?
- What are the roles of molecular imaging, fluciclovine (Axumin), prostate-specific membrane antigen (PSMA), and choline?
EVANS: We have the Axumin PET [positron emission tomography] scan, and I must admit that it seems our local radiologists are not as adept at reading them as your radiologists [may be]. I’m not sure that a PET scan with Axumin, in our neck of the woods, would be something that I would order here. But I can see why some [practitioners] might want to order it.
BEED: We like to do genetic testing. It’s interesting what comes up, especially with this family history. Sometimes, genetic testing comes up that’s positive but has no meaning.
SMITH: What jumped out in the history that prompted you to want to do germline testing in this patient?
BEED: The breast cancer and the pancreatic cancer history, 2 different tumors that were positive [in his family history].
SILVA: It could be used for later. I think the approval for olaparib [Lynparza] is for metastatic disease, not for biochemical relapse. But it’s good to have in your pocket, [to address] each way when the patient develops metastatic disease.
SMITH: You’re right, it wouldn’t be actionable now. There’s the issue of both germline testing as well as next-generation sequencing of tumor, either tumor biopsy or blood-based testing. They’re complementary tests. In a patient [such as] this, I’d certainly be inclined to do germline testing, of course, because it’s binary: either he has it or he doesn’t. Then, even if it’s not immediately actionable, you have that information in your back pocket when you make future decisions about his care.
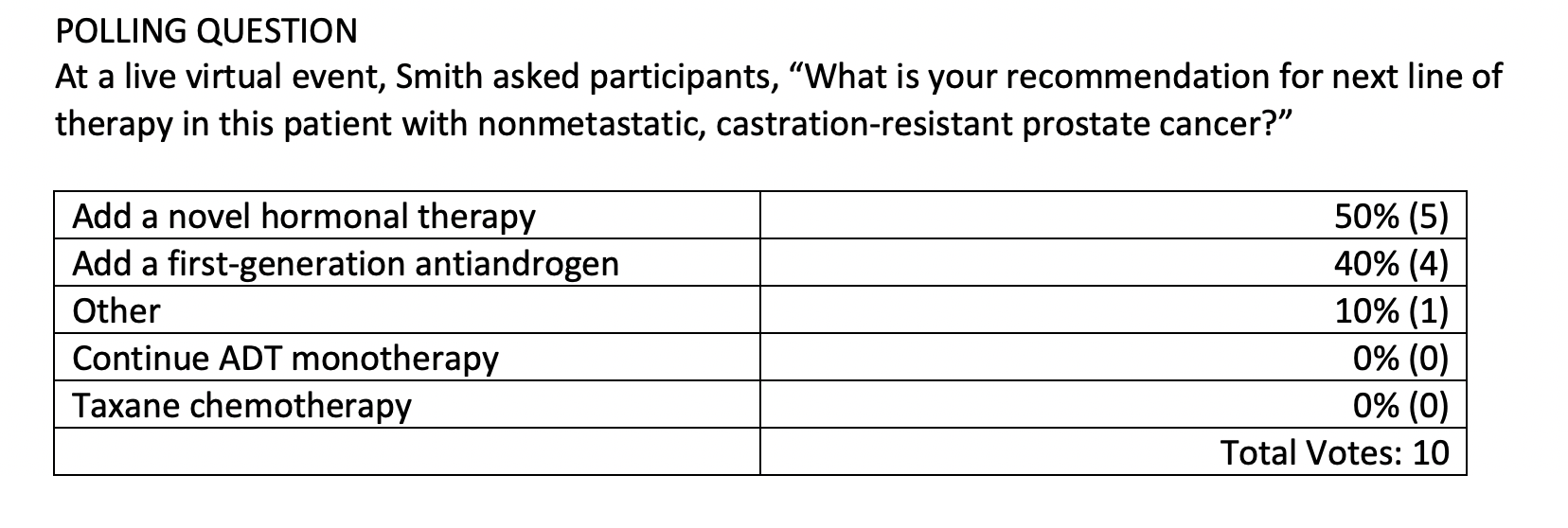
MAMADGI: I was looking at apalutamide [Erleada] or darolutamide [Nubeqa] because this [patient has] nonmetastatic, castration-resistant prostate cancer (CRPC) with a PSA-DT of less than 10 months, and I think there’s an indication for that.
SMITH: [The terminology is a bit confusing.] Novel hormonal therapy is a term that is sometimes used to include those 2 drugs that you mentioned, as well as enzalutamide [Xtandi] or abiraterone [Zytiga]. Basically, all the newer approved androgen receptor pathway inhibitors are sometimes called novel hormonal therapies. They’ve been approved for a few years, so I don’t consider them so novel anymore, but the terminology’s quite confusing. There’s no shortage of terminology for these drugs.
RAJ: I thought apalutamide is indicated for nonmetastatic prostate cancer, acting like a biochemical failure indication.
SMITH: You’re exactly right. As I was mentioning, the terminology is a bit confusing. Novel hormonal therapy is a commonly used term to include apalutamide, enzalutamide, darolutamide, and abiraterone. [It’s] not very specific and I don’t consider them so new anymore, but you’re exactly right. There are 3 drugs that are approved in nonmetastatic CRPC based on improved metastasis-free survival [MFS] and overall survival [OS] in placebo-controlled trials.

SMITH: Apalutamide, darolutamide, and enzalutamide are specifically approved in a patient [such as] this, based on the results of completed phase 3 trials of a similar design. Abiraterone acetate is not approved in this setting. It was studied in a randomized phase 2 trial, but that never led to regulatory approval.
BIERENBAUM: I picked enzalutamide, and it’s my favorite [option in this setting], just because I have the most experience with it and the most comfort giving it. After I marked that, though, I [remembered] there’s a history of seizures, so I probably would take that back if I had the opportunity.
SMITH: It’s not a wrong answer, and I think the [reasoning] that you described is rational. Enzalutamide has a broad regulatory approval—the broadest of the 3 drugs, it’s approved in the metastatic hormone-sensitive disease, nonmetastatic CRCP, and metastatic CRPC; it covers all those bases. As you correctly pointed out, it does have this risk of seizures, as is the case for apalutamide, which is structurally very similar, and both of those drugs also have a risk of falls and fractures. [Although] there have been no head-to-head trials, darolutamide does not cross the blood-brain barrier and appears to have some safety advantages, including absence of excess risk of falls or fractures, and it is not known to cause seizures. In general, I would say any of those 3 choices would be good. In this specific patient, who had a history of seizures, I would not be comfortable prescribing either apalutamide or enzalutamide. My pick was darolutamide.
EFIOM-EKAHA: I chose darolutamide for the same reason you just mentioned. It doesn’t cross the central nervous system, the blood-brain barrier, and therefore it causes less fatigue and less incidence of falls and fractures. All 3 of the choices, apalutamide, darolutamide, and enzalutamide, show survival benefit. But darolutamide seems to have fewer [adverse events].
SMITH: For the average patient, maybe you can make a good case for any of the 3 drugs, but for patients with a seizure disorder, you’d want to pick darolutamide. For older, frail patients, I like darolutamide because of the lower risk of falls and fractures. The other thing…is the differences in drug to drug interactions, and darolutamide has the least of those. Whenever I’m prescribing the other 2 drugs, I routinely get out my drug interaction checker, because there are quite a few things that you need to consider in that regard, [such as] a lot of medicines that are commonly prescribed in this patient population and age.
EVANS: Could you comment on the number of pills that the patients has to take in a day [with each of these drugs]? Does that make a difference?
SMITH: Enzalutamide is 4 tablets once a day. Darolutamide [has] somewhat larger pills and twice a day, so that would be considered less convenient for a patient.
SILVA: The question is, if you choose drug A, what’s the rule of drug B and drug C? Does the activity of 1 of the other 2 [affect your decision] if the patient doesn’t respond to the first treatment?
SMITH: There’s nearly complete cross-resistance between those 3 antiandrogens. The same would be true if you add in abiraterone, which, although it is a different mechanism of action, is not an antiandrogen. It’s an angio biosynthesis inhibitor. There’s a very high rate of cross resistance….There’s an occasional response when you do the switch, but [responses] tend to be very brief and we’re loathe to even do that because there’s very little in it for the patient, with few exceptions. It does matter, right? For an individual patient, it provides an opportunity to tailor the traits of the drug to the patient, based on their specific age, health, comorbidities, and other medications. There are certain patients, perhaps such as the [patient in this case] where there’s going to be a clear [treatment to choose] because of his medical history. There’s not as much in it for the patient for the second drug because there’s very little benefit seen there.
The only time I generally do the switch is going to be in [a patient] who discontinued early because of intolerance. The classic [example] would be someone who started enzalutamide, had profound fatigue, and couldn’t tolerate the drug. But you never really know if they [would have] responded because they were on it for too short of a time. In that case, I would happily switch them to an alternative drug such as abiraterone, but that’s because there are really no concerns about the cross resistance because they didn’t fail the first drug yet.
CASE SUMMARY
After shared decision-making, darolutamide was initiated in this patient.
DISCUSSION QUESTIONS
- What are your thoughts on the choice of darolutamide?
- What agents are FDA-approved for the treatment of nonmetastatic CRPC ?
- Does the patient’s history of seizures affect your choice?
- What if the PSA-DT were 15 months?
- How does darolutamide differ from other agents in its class?
- What do you think about its toxicity and tolerability?
PATHE: I would still recommend the darolutamide.
SMITH: [In regard to the PSA-DT], less than 10 months was part of the eligibility criteria [for the SPARTAN (NCT01946204) trial that looked at darolutamide in this population], and the median PSA-DT in the enrolled subjects was [approximately] 5 months. However, that label doesn’t specify anything about PSA-DT. I’ve often been asked about that, why is that the case? Why did the FDA give a pass to the manufacturers without requiring that in the label? Really, it’s because the FDA, when they include biomarkers in labels, they need them to be robust and reproduceable. The challenge with PSA-DT is it’s not a measurement, it’s a calculation. It could come from PSAs measured at 3 different laboratories and all those sorts of things. So the FDA, I think appropriately, elected not to include it. But it’s something I think about when I’m making decisions. It’s not binary—a patient with a PSA-DT of 9 months isn’t really that different than a patient with PSA-DT of 11 months—but it is something to think about. [At least,] in terms of timing of treatment and how pressing it is to intervene and whether a patient with a slow DT might be appropriate for observation, at least initially.
EVANS: I’ve used the low-dose [250 mg with food] abiraterone and prednisone regimen on occasion, and I wondered what your thoughts were about that as opposed to the regular dose. And do you use that regimen? Would that provide a better argument for using one of the other options in that setting?
SMITH: Early on, when it was first approved, it was challenging because often it wouldn’t be reimbursed or the co-payments were very high for patients. So for financial reasons only, I have prescribed patients the 250 mg dose with food. It’s better absorbed, but the absorption is highly variable with food, so I don’t like doing it because I’m more concerned about safety in that setting. I haven’t done it in years, mostly because we’ve managed to get the patient’s drug covered through foundations and other things such as that. I haven’t, but if I wasn’t able to do it in some other manner, meaning that there were financial considerations, I would consider doing it.
EVANS: I’ve had a couple [instances] lately where you use the low dose and [patients] progressed on it, and I wondered whether the efficacy of the low dose is equivalent to the usual higher dose, whether that would make changing to one of these other drugs a little more appealing, perhaps?
SMITH: The efficacy has not been compared. What was studied in that small, randomized trial that was published was pharmacokinetics/pharmacodynamic parameters. It didn’t prove noninferiority, it just showed that you could achieve similar drug levels with the low dose when given with food. That is so far from proving equivalence that I reserve that approach for patients where there’s no other convenient or financially feasible way.
BEED: Sometimes you wait and watch if it’s a longer time because they’re older, and maybe they have other problems, and finances do get to be a problem. I’m just asking if you’re familiar with that or anything about the cost of these drugs, compared with what people can afford, because it’s hard to get funding for patients sometimes.
SMITH: I think it’s important to acknowledge all these drugs we’re talking about are very expensive. The co-pays are going to vary, and the financial toxicity of these drugs can vary. At least, it seemed in my patient population that the patients are very proud, even when it’s really a big impact on their finances; they’re often not willing to talk about that. It is something worth them taking on directly, just so you get a better sense for [adherence] and related issues that can be a complication of financial toxicity.
DISCUSSION QUESTIONS
- What are the practical implications of having both OS and metastases-free survival data for androgen receptor–targeted therapy in nonmetastatic CRPC?
- Does this influence your use of these agents?
- How will novel imaging affect your approach?
- Do patients with PSMA-only metastases fit into this group or do they have low-volume metastatic disease?
MAO: Is there any difference between OS in the United States versus OS outside of the United States that speaks to drug access in the following lines?
SMITH: OS was demonstrated in a placebo-controlled trial. But is that applicable in the United States, where we have access to lots of other lines of therapy? For survival, there are fewer events, so I don’t believe we’ve yet reported forest plots for survival. Perhaps the more important issue is that when we designed SPARTAN, we wanted it to be generalizable, and we also wanted to see that patients stayed on the trial until they met the primary end point.
It’s a placebo-controlled trial. If they came off before they progressed, they provided no meaningful information, so to provide incentive for them to stay on. And, particularly in places whether they might not otherwise have access to a subsequent therapy, patients could receive abiraterone acetate for free, provided by the study. That was the most-used subsequent therapy. At the time of the analysis for OS, [approximately] 80% of patients in the placebo group had received subsequent life-prolonging therapy. Far and away, the most common drug was abiraterone, based on the study design.
The rates of subsequent therapy were also high in the other 2 trials, but not quite that high because they didn’t have this additional provision. I guess that’s the strongest argument to say that the OS benefit would be applicable, including in places such as the United States where there’s access to subsequent therapy. In other words, placebo patients who develop 1 metastasis or [something similar to that] and met the end point could immediately get abiraterone, and that occurred at a high rate. That’s probably the best way to answer your question. There are no meaningful geographic differences in the outcome for MFS. I don’t know that we generated forest plots for OS, in part because there were far fewer deaths than MFS events, so we might have not had the statistical power to do that. If it was done, there were no differences by geography. We probably, at least in SPARTAN, eliminated that by providing abiraterone as part of the trial.
Reference
Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2020 Jun 4;382(23):2197-2206. doi:10.1056/NEJMoa2003892
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More