Primary Chemotherapy Before Surgery in Ovarian Cancer May Be Preferable
Results of the CHORUS trial confirm the noninferiority of primary chemotherapy followed by surgery versus primary surgery and then chemotherapy, and show that performing chemotherapy first is associated with less morbidity and mortality in patients with newly diagnosed, advanced ovarian cancer.
Lancet,1confirm the noninferiority of primary chemotherapy followed by surgery versus primary surgery and then chemotherapy, and show that performing chemotherapy first is associated with less morbidity and mortality in patients with newly diagnosed, advanced ovarian cancer.
Explaining the need for this trial, the authors state that although it has been found that primary chemotherapy led to improved debulking and reduced surgical complications in observational studies, two meta-analyses of nonrandomized studies came to conflicting conclusions regarding the impact of delaying surgery on survival.2,3
CHORUS
This phase III noninferiority controlled trial randomized 276 patients to primary surgery followed by chemotherapy and 274 patients to primary chemotherapy followed by surgery. The women in the primary surgery group underwent debulking surgery and then 6 cycles of primary chemotherapy. Women assigned to the primary chemotherapy group were given 3 cycles of chemotherapy (after laparoscopic or image biopsy, fine needle aspiration or other confirmation of diagnosis) followed by surgery and then 3 cycles of chemotherapy. Chemotherapy was carboplatin AUC5 or carboplatin AUC6 with paclitaxel 175 mg/m2, or another carboplatin combination or carboplatin monotherapy. Follow-up was monthly for 9 months, then quarterly for 2 years, biannually for 3 years and then annually until study end, withdrawal, or death.
Quality of life was measured with the European Organization for Research and Treatment of Cancer, core questionnaire (QLQC-30) and the QLQ-OV28 questionnaire, which is specific for ovarian cancer.1
The primary endpoint was overall survival (OS), defined as the time from randomization to death. Progression-free survival (PFS), defined as the time from randomization until first progression or deathwhichever occurred first—and quality of life were the secondary outcomes. Adverse events were monitored at chemotherapy and after.1
Patients Characteristics
The study recruited patients in the United Kingdom and New Zealand between March 1, 2004, and August 3, 2010. The paper reports data as of May 31, 2014 (data cut-off). The median age of patients was 65 years, and the majority had FIGO-defined grade III or IV ovarian cancer. Over 70% of the tumors in either group had histology classed as high-grade serous, and over 70% were poorly differentiated. More than 99% primary surgery and 98% primary chemotherapy patients had a cancer antigen 125/carcinoembryonic antigen ratio >25.1
Outcomes
During the study, the majority of deaths were attributed to ovarian cancer, and the median duration of follow-up for the 99 patients who were still alive at cut-off was 4.4 years. Median OS for each group (intent-to-treat population [ITT]) is shown in Figure 1. The hazard ratio was 0.87 in favor of patients in the primary chemotherapy group (upper bound of the one-sided 90% CI 0.98 [95% CI 0.72-1.05].1
Figure 1. Median overall survival for CHORUS study.
Figure 1. Median overall survival for CHORUS study.
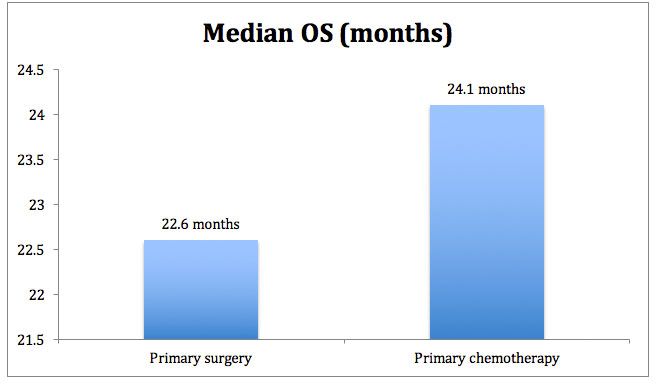
The 3-year survival rate was 32% and 34% in the primary surgery versus primary chemotherapy groups respectively.1
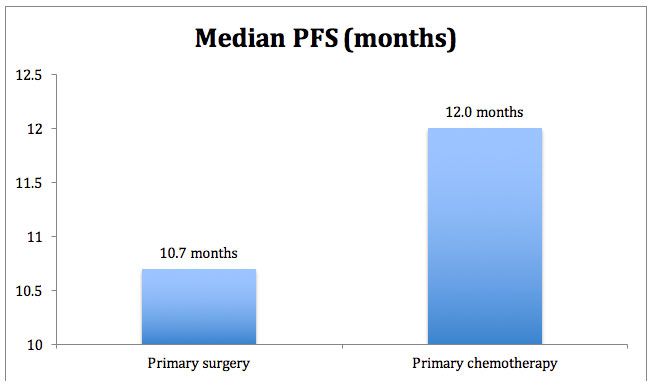
The results for PFS are shown in Figure 2, again greater for the primary chemotherapy group versus the surgery group. Hazard ratio for PFS was 0.91 (95% CI 0.76-1.09).1
Figure 2. Median progression-free survival for CHORUS.
Figure 2. Median progression-free survival for CHORUS.
The authors also examined data for the per-protocol population and found similar trends. Median OS was 23.7 months versus 25.8 months for primary surgery versus chemotherapy, and the HR was 0.89, favoring the primary chemotherapy group (95% CI 0.73-1.08). Other survival data were similar to that for the ITT population. Analysis confirmed that no subgroup (age, stage, tumor size, performance status, chemotherapy at baseline) fared better with primary chemotherapy.1
There was a marked difference in residual disease. Residual disease was debulked to less than 1 cm in 41% versus 73% of patients in the primary surgery versus primary chemotherapy groups respectively (P= .0001). Debulking achieved a status of no macroscopic disease in 17% versus 39% of patients in the primary surgery versus primary chemotherapy groups respectively (P= .0001). In the quality of life analysis, more patients in the primary chemotherapy group versus the surgery group had an improvement of ≥5 points at the 6 and 12 month time points, but this was not statistically significant.1
Patients who underwent primary surgery experienced more grade 3 or 4 adverse events (AEs) than the primary chemotherapy group at 24% versus 14% (P= .007). Patients in the primary chemotherapy group had shorter hospitalization times following surgery than the primary surgery group (P< .0001). Deaths within 28 days of surgery were higher in the primary surgery group at 6% versus <1% in the chemotherapy group (P= .001). A familiar and known AE profile associated with the chemotherapy was experienced by each group.1
What this Means for Patients
The authors write that the CHORUS study clearly shows that primary chemotherapy followed by surgery in patients with advanced ovarian cancer and a poor performance status leads to similar overall survival as patients given primary surgery followed by chemotherapy. They have established that the primary chemotherapy approach is noninferior to the primary surgery approach. They point out that the debulking data favoring the primary chemotherapy approach was very similar to the finding of the EORTC trial 55971.4The added value of the primary chemotherapy approach is that it is associated with less mortality and morbidity and a trend toward a better quality of life for patients. This suggests that the primary chemotherapy approach is a valid treatment option for these patients.
References
1. Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial.Lancet. 2015;386:249-257.
2. Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis.Gynecol Oncol. 2006;103:1070-1076.
3. Kang S, Nam BH. Does neoadjuvant chemotherapy increase optimal cytoreduction rate in advanced ovarian cancer? Meta-analysis of 21 studies.Ann Surg Oncol. 2009;16:2315-2320.
4. Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer.N Engl J Med