Pemmaraju Reviews a Patient Case With Myelofibrosis Following a Targeted Tweet Chat
During a Targeted Oncology tweet chat, Naveen Pemmaraju, MD, and Aaron Gerds, MD, MS, led a discussion on a patient case with myelofibrosis. They reviewed the options from a Twitter poll and how they would approach treatment of this particular patient.
Naveen Pemmaraju, MD

Naveen Pemmaraju, MD
During aTargeted Oncologytweet chat, Naveen Pemmaraju, MD, and Aaron Gerds, MD, MS, led a discussion on a patient case with myelofibrosis (MF). They reviewed the options from a Twitter poll and how they would approach treatment of this particular patient.
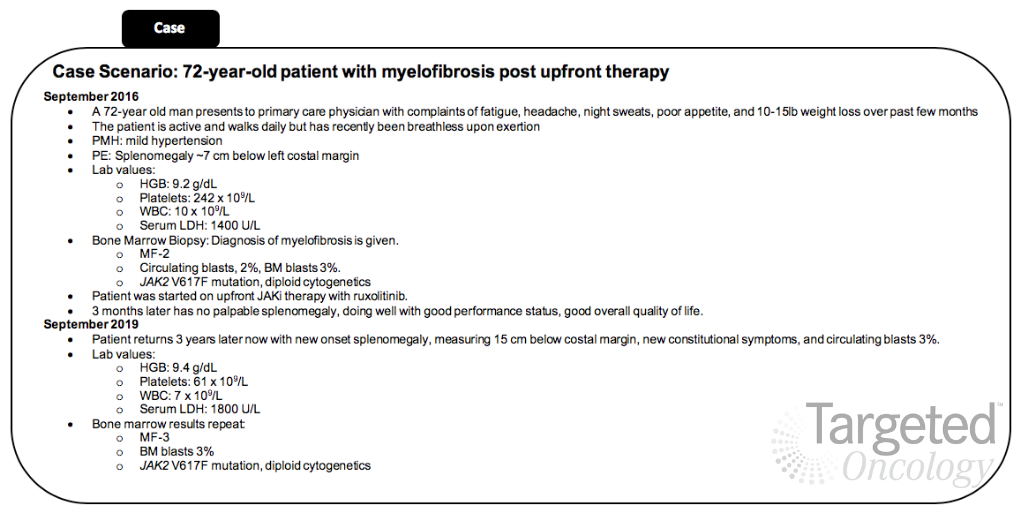
The patient evaluated in the tweet chat presented with MF to his primary care physician in September 2016 at 72 years old. He was treated with frontline ruxolitinib (Jakafi), a JAK inhibitor, but presented with disease progression 3 years later.
There have been very limited treatment options for patients with MF outside of JAK inhibition with ruxolitinib. However, in August 2019,the FDA approved fedratinib (Inrebic) for the treatment of patients with intermediate-2 or high-risk primary or secondary MF. This agent provided a new option for patients with MF. Although the approval has provided new opportunities in this space, oncologists are still evaluating where this agent should fit into the treatment landscape with other therapies, including interferon therapy or stem cell transplant (SCT).
POLL: How would you treat this patient at this point?#TargetedCaseChat#MPNsm#Myelofibrosis
Targeted Oncology (@TargetedOnc)October 7, 2019
“These choices are interesting and all plausible in this case,” said Gerds, assistant professor of medicine in the Hematology and Medical Oncology department at the Cleveland Clinic Taussig Cancer Institute, during the tweet chat. “There is some preclinical and retrospective data to suggest that interruption and re-treatment with [ruxolitinib] works.”
Although continuing ruxolitinib and clinical trials had a fair amount of interest, according to the poll, the majority of votes were for switching to fedratinib. However, Pemmaraju and Gerds leaned more toward putting the patient on a clinical trial.
“Fedratinib has been shown to work in patients previously treated with [ruxolitinib], especially those with lower (50-100) platelets (perhaps),” Gerds tweeted. “We are always trying to do better by our patients, so clinical trials rise to the top for me.”
Overall, Gerds concluded with 2 key points. First, JAK inhibitors work, particularly in patients with symptomatic patients with preserved counts. Secondly, he noted that a second JAK inhibitor, such as fedratinib, could be used after the first JAK inhibitor fails, but physicians should consider why the original agent failed in order to select second-line treatment.
In an interview withTargeted Oncologyfollowing the tweet chat, Pemmaraju, an associate professor in the Department of Leukemia at The University of Texas MD Anderson Cancer Center, reviewed key discussion points from the tweet chat and Twitter poll. He highlighted different treatment options for patients with MF, as well as some upcoming research to be presented at the 2019 ASH Annual Meeting.
TARGETED ONCOLOGY: What considerations would you make when you first approach a patient like this one?
Pemmaraju:This hypothetical patient case is actually becoming more common in the myeloproliferative neoplasm (MPN) clinic. It’s important to start thinking about options after JAK inhibitor therapy, mainly because many of these options were not around 5 years ago. I think the first principle to think about in any patient who is failing the standard of care in these rare blood cancers, MPNs included, is to go for a clinical trial. A clinical trial offers you the chance to give something novel as a treatment opportunity for the patient in this era where we only have a few drugs approved and not many drugs widely available. I think in this day and age, after a JAK inhibitor, clinical trial remains the gold standard in terms of what you would offer to a patient next.
TARGETED ONCOLOGY: What is there to know about clinical trials in this space right now?
Pemmaraju:In the real-world, it’s true there are barriers that we must break down together for access to clinical trials because in reality, less than 10% of oncology patients are enrolled on clinical trials. This is something we all have to improve on. Some of these barriers in our patient population can include older age of the patient and comorbidities that prevent travel; lack of resources, financial and otherwise; transportation logistics to be able to get to an academic center; distance to the academic center; and then of course, availability and eligibility of the clinical trial itself. Even recognizing all of these barriers, I still think that access to or at least discussion of clinical trialwhich can be facilitated online by clinicaltrials.gov, run through the National Institutes of Health, or through other portals such as social media, online, and other areas of critical important information—at least gives the patient and the provider some opportunity to think about that as an option.
Within clinical trials, particularly for this patient case, you have options. You have the JAK inhibitor combination option, which is either [in the setting of a] suboptimal response or in the frontline setting. This is where you take the JAK inhibitor and any clinical trials combining [this drug] with any number of agents, such as hypomethylating agents or other novel agents. That is 1 pathway.
The second pathway is completely novel agents beyond JAK inhibitors. There is a whole host of drugs that are all publicly available, all these clinical trials have registered numbers, and so that is a whole subclass of drugs that are out there that are different from the JAK pathway. I just wanted to put this out there as an option, but I still believe very strongly that in 2019 and beyond, a clinical trial is your best option in many, if not all, of our patients.
TARGETED ONCOLOGY: What other options are available outside of clinical trials?
Pemmaraju:There are many other JAK inhibitors out there that give you another opportunity for treatment. That is where this patient case comes in. Breaking news in the last few months, there is a second JAK inhibitor that was just approved, known as fedratinib. This is an inhibitor of JAK2, and it also has activity against bromodomain and other pathways. It was approved in August 2019 in intermediate- to high-risk MF. It appears to be a somewhat broad approval label in that first-line versus second-line is not specified. The dose is 400 mg once daily. There are some parameters and restrictions on the dose, including a black box warning for encephalopathy, particularly watching out for Wernicke's encephalopathy, which may have been seen in some of the cases in clinical trials. There is a requirement of measuring thymine levels as part of the package insert and the approval.
I think 1 interesting thing is that the dosing does come down to platelet counts, all the way down to 50 and above, just like ruxolitinib. Although here with the case of the newer drug, it looks like the dosing can be maintained at the full dose of 400 mg, whereas ruxolitinib has the dosing parameters based on platelets.
TARGETED ONCOLOGY: What data supported the approval of fedratinib for patients with MF?
Pemmaraju:This new drug, fedratinib, is approved based on the phase III study known as the JAKARTA study, which took patients with intermediate- to high-risk MF and platelets of 50 and higher. There were actually 3 arms, including an arm with fedratinib at 400 mg and 1 arm of fedratinib 500 mg. Primary endpoints were spleen volume reduction by 35% or greater at 24 weeks and symptom burden reduction. This did meet its primary endpoint.
There was a second study known at JAKARTA2 that evaluated fedratinib in patients with MF after ruxolitinib. This was 97 patients with intermediate-2 to high-risk MF, again with platelets of 50 or higher. They met some definition of intolerance or resistance to ruxolitinib, and the endpoints appeared to be met. About half of the patients had some form of gastrointestinal toxicitydiarrhea, nausea, and vomiting, usually in the lower grades. Like any JAK inhibitor, whether it’s ruxolitinib or fedratinib, they all have some element of myelosuppression, so you have to watch for anemia or thrombocytopenia. There was a reanalysis presented at the 2019 ASCO Meeting, which used a more stringent definition of ruxolitinib failure for this JAKARTA2 trial, and even with the stricter definition, the majority of patients (81%) met the criteria and still had what was thought to be a meaningful reduction in spleen volume or symptoms.
On this basis, the drug is approved. It’s out there, and it can represent a nice option for the eligible and appropriate patient in the setting of either frontline or failure after ruxolitinib.
TARGETED ONCOLOGY: How do fedratinib and ruxolitinib compare? What would be the rationale for continuing ruxolitinib in this patient?
Pemmaraju:In terms of other options, before fedratinib was approved and in the absence of a clinical trial, we had very limited options. There are some data from Dr Aaron Gerds on this idea of re-challenging ruxolitinib. Sometimes when you have these limited options, you must be creative with those options. There are some limited data coming in that perhaps changing the dose, continuing the drug, or re-challenging with the drug after an absence of being on the drug, may still have some benefit. That field and that area needs further investigation, but that is something of interest to consider. That is called ruxolitinib or JAK inhibitor re-challenge, or redosing.
TARGETED ONCOLOGY: What other therapies are of importance to highlight with this patient case that were not included in the poll?
Pemmaraju:Another area that I think is of high importance in the MF field as we move forward and is of continued importance is SCT. I think anytime we have 1 of these case discussions, and we are talking about novel therapies, we [should always] talk about allogenic SCT. One of the important discoveries or tools that we have are 2 new scoring systems. Yes, our field has a lot of scoring systems and we continue to get new ones every year, but the 2 I would like to highlight that are very helpful in this patient case are MIPSS70 or MIPSS70+. This was published in theJournal of Clinical Oncologyin 2018, and basically this was the first scoring system to include fibrosis grade, some of the molecular mutations, and it is designed to help see which of the patients are at higher risk with some of the newer features, molecular mutations, and fibrosis to determine who might be a candidate for transplant.
In addition to that, another scoring system came out, called the Comprehensive Clinical Molecular Transplant Scoring System for MF undergoing transplant. This was published inBloodin 2019, so it’s very recent. Again, there is a nice mix of clinical factors, such as age and performance status, but it also includes molecular mutations, such as ASXL1,and the type of donor, to again try to prognosticate which patients might benefit from transplant.
Essentially what we are seeing is that the majority of these scoring systems agree that patients with intermediate- to high-risk disease, good performance status, and moderate to younger age may be the ones that would most likely benefit, but there is also this area of investigation in patients with low- to intermediate-risk disease, such as intermediate-1 but with higher risk features, such as molecular mutations and others, if they should also be considered for allogenic SCT or if this should become an easier part to facilitate in the therapy program. Allogenic SCT in 2019 and beyond, it’s very important as we consider therapies. This is our curative therapy to date.
TARGETED ONCOLOGY: Only 6% of people in our poll were interested in switching this patient to interferon therapy. What would be the pros and cons to interferons for this patient case?
Pemmaraju:Interferon is actually the oldest of the drugs we have. SCT and interferons have actually been around longer than the novel agents. They represent the first “immunotherapies” in the blood cancer field. Interferon has a long history in the MPN space, particularly with the early treatment of chronic myeloid leukemia (CML) and of course still in the modern-day treatment of polycythemia vera and essential thrombocytopenia formulation overtime, including the current 1 we use most widely in the United States, pegylated interferon abrasion. Also, there is a very important novel formulation called ropeginterferon that is being developed in Europe and is hopefully coming into more clinical trials here.
There are some studies that have shown there is benefit possibly to using interferon in patients with MF, particularly in those with early MF and prior to going to the later stages of more advanced disease. Interferon, in some of its different formulations, can be thought of as a potential treatment for some of these patients with early MF. Of course, a lot of this is best done in clinical trials, but because interferon is available off-label, it’s something that gets discussed.
I would also like to highlight that there are a couple ongoing trials both in Europe [and the United States] that are featuring the combination of ruxolitinib and interferon in MPNs in general. One is the COMBI study, a phase II evaluating 18 patients with low- to intermediate-risk MF. It looks like preliminary safety has been established with a decline inJAK2V617F allele burden. There is also a second study, Ruxopeg, a phase I/II study, that has also concluded a phase I with no dose-limiting toxicities currently observed there. The phase II had also shown some decrease in JAK2V617F allele burden overtime. This combination approach, of course, is very new and must be done in the clinical trial setting, but there is a long history of interferon both as a single-agent and now moving into the combination era in not only the earlier stage of MPNs but also possibly even in MF. I would say to stay tuned, let’s continue to follow these studies and see if there are any other studies in the United States and other areas to further confirm and validate these studies.
TARGETED ONCOLOGY: What other novel agents are under investigation right now that we can look forward to seeing more data on at future meetings?
Pemmaraju:There are, and I am the principal investigator for a couple of these clinical trials, but there are some of these agents out there. At the 2018 ASH Meeting, my group presented data on LCL-161, which is a SMAC mimetic or inhibitor of apoptosis protein (IAP) antagonist. This is an ongoing phase II trial presented last year that showed a response rate of 25% to 30% in patients primarily with relapsed/refractory MF. That’s a novel area of investigation.
Several of my colleagues are involved in studies with the combination of JAK inhibitors and BCL-XL inhibition, which is an ongoing trial. Hsp90 inhibition is in ongoing trials. Targeting CD123 with tagraxofusp-erzs (Elzonris) is an area of interest that I have been involved in. That is the drug that gained FDA approval in a rare leukemia subtype called blastic plasmacytoid dendritic cell neoplasm and is now being tested in a variety of other CD123-expressing tumor types, such as MF, CML, and others. Some of my colleagues have been investigating the MDM2 pathway, the idasanutlin (RG7388) and other agents targeting fibrosis, cytokines, and other pathways with the PRM-151 drug, TGF-beta modulation, sotatercept, luspatercept, aurora kinase A inhibition, bromodomain inhibition, LSD1 inhibition, and so on and so forth. There are many active clinical trials that are in the public domain. All of these can be accessed on clinicaltrials.gov, which I think is a great resource. Another drug we’re looking at is imetelstat, a telomerase inhibitor, and this has been presented at ASH. These are just a selection and not meant to be a comprehensive list.
We have the 2019 ASH Meeting quickly approaching, and I am eager to see what new phase I and phase II studies, both in the JAK inhibitor domain and outside the JAK inhibitor domain, will be presented. There is 1 thing to note in the JAK inhibitor space; besides ruxolitinib and fedratinib, we are eagerly awaiting results for pacritinib and momelotinib. Pacritinib, of course, will be entering into the phase III with the PACIFICA trial, while momelotinib is planned for a phase III with the MOMENTUM trial. Both of those will be, evidently, randomized studies, and there are a host of other JAK inhibitors [in development]. It is an exciting time in the field on behalf of our patients to see what exactly is going to be presented at the new ASH meeting, and then what momentum that carries into 2020 with JAK inhibitors, novel agents, and combinations.
TARGETED ONCOLOGY: What do you hope the community takes away from this discussion?
Pemmaraju:I think the key takeaways in 2019 and beyond is, in rare cancers such as MF, information and knowledge are key for the patient, the loved one, the caregiver, and the provider. If you’re in a community practice and you only see a couple of these patients per year or only a couple during a career, I think referral to a clinical trial still is the top aspect that you can use. [I recommend] co-management with an academic center and getting multiple opinions. I always say that I think for the patient, the providers, and even all of us in academic centers, I frequently obtain and seek second and third opinions from my own colleagues.
In rare diseases where there is little evidence and very few drugs, refer for clinical trials. Also, be aware that there are other JAK inhibitors that are in development besides ruxolitinib. Thirdly, be aware that there is a host of novel agents outside of the JAK pathway that are being tested in phase I/II clinical trials all over the country that may be an option for some selected patients, if they’re appropriate.
TARGETED ONCOLOGY: What other tools would you recommend to the community oncologist for keeping up with the latest data in the field of MPNs?
Pemmaraju:I continue to find social media and particularly Twitter to be a great resource for both the contribution of information in rare blood cancers and to learn. As I progress in my social media influence, I really am learning a lot myself. For me, by checking #MPNsm on social media, which is something my colleagues and I created at a grassroots level 4 or 5 years ago, it really allows me to follow the field at a very close level. [There is] very little spam, it’s not regulated. It’s a self-regulated Twitter medical community that arose, inspired by the Healthcare Hashtag Project. I think it’s a great way to follow along with experts and key thought leaders on a daily or weekly basis. It’s also a quick and easy way to contribute, bringing in new papers in the field or new angles, and when we are at these conferences like ASH, ASCO, EHA, AACR, and some of the other regional conferences, it’s an awesome way to communicate the information that is brought up within the meeting. I think that is a great way to get real-time information, thoughts, debates, and discussions whether you are at the meeting or remotely following along somewhere else. I think Twitter and social media for our MPN field has amplified the ability to gain and contribute information. Of course, we all have to remember in this media era, we must honor privacy and other regulations of media as we all learn together in this new era.
Disclosures: Naveen Pemmaraju, MD
I. Consulting/honorarium:Celgene; Stemline; Incyte; Novartis; MustangBio; Roche Diagnostics, LFB
II. Research funding/clinical trials support:Stemline; Novartis; Abbvie; Samus; Cellectis; Plexxikon; Daiichi-Sankyo; Affymetrix
III. Grants/funding:Affymetrix, SagerStrong Foundation
IV. Board Memberships (non-compensated):
a. Dan’s House of Hope (DHOH) Board of Directors, non-compensated/volunteer work
b. HemOnc Times/Oncology Times, Board Member: Editor-in-Chief HemOnc Times (non-compensated/volunteer work