Palbociclib Demonstrates Prolonged OS/PFS When Initiated in Metastatic Breast Cancer
Patients who received palbociclib and fulvestrant before chemotherapy for metastatic breast cancer had greater clinical benefit versus patients who received placebo and fulvestrant, according to an exploratory subgroup analysis of the phase 3 PALOMA-3 trial.
Hope S. Rugo, MD, FASCO

Patients who received palbociclib (Ibrance) and fulvestrant before chemotherapy for metastatic breast cancer had greater clinical benefit versus patients who received placebo and fulvestrant, according to an exploratory subgroup analysis of the phase 3 PALOMA-3 trial (NCT01942135).1
The post hoc analysis demonstrated an improvement in overall survival (OS) with the addition of palbociclib and fulvestrant compared with placebo and fulvestrant across different patient subgroups. Findings were presented during the 12th European Breast Cancer Conference, held virtually on October 2 and 3, 2020.
“The analysis evaluated the safety of palbociclib in patients with or without chemotherapy who were stratified by menopausal status in the overall and endocrine-sensitive populations in PALOMA-3,” said lead study author Hope S. Rugo, MD, FASCO, who is a professor of medicine and director of Breast Oncology and Clinical Trials Education at the University of California San Francisco Helen Diller Family Comprehensive Cancer Center, during a presentation of the data.
Previously Reported PALOMA-3 Results
In PALOMA-3, patients with hormone receptor–positive, HER2-negative advanced breast cancer were randomized 1:1 to receive either palbociclib or matching placebo plus fulvestrant. Patients were eligible for enrollment regardless of whether they were pre/ perimenopausal or postmenopausal. They could have received up to 1 line of prior chemotherapy for advanced disease and had disease progression on at least 1 line of endocrine therapy. Patients were stratified by the presence or absence of visceral metastases, sensitivity to prior endocrine therapy, and menopausal status. The primary end point of the trial was investigator-assessed progression-free survival (PFS).
Previous results showed that median OS in the intention-to-treat population was 34.9 months for patients treated with palbociclib and 28.0 months for placebo (HR, 0.81; 95% CI, 0.64-1.03; P = .09), for an absolute difference of 6.9 months. These results did not reach statistical significance.2
Median OS was longer in patients with endocrine therapy–sensitive disease, with a median OS of 39.7 months in the palbociclib arm and 29.7 months in the placebo arm (HR, 0.72; 95% CI, 0.55-0.94), for an absolute difference of 10.0 months.
Prolonged PFS and OS
The subgroup analysis reported at the meeting sought to determine whether baseline characteristics, such as menopausal status and prior treatment with chemotherapy, affected the OS benefit observed in PALOMA-3. Patients who received palbociclib plus fulvestrant had prolonged PFS compared with those receiving placebo plus fulvestrant in all subgroups of patients, regardless of whether they had received prior chemotherapy. This was observed in both the overall and endocrine-sensitive populations.
“Regardless of treatment, patients without prior chemotherapy had longer progression-free survival than patients with prior chemotherapy in the treatment arm,” Rugo said. Baseline characteristics of the patient population revealed that about one-third of patients who were enrolled in PALOMA-3 had received prior chemotherapy for advanced disease. “In the palbociclib-plus-fulvestrant group, patients with endocrine-sensitive disease had a slightly longer progression-free survival than the overall population,” she said.
Median OS in patients without prior chemotherapy in the intention-to-treat population who were treated with palbociclib and fulvestrant was 39.7 months compared with the placebo arm at 29.5 months (HR, 0.75; 95% CI, 0.56-1.01). In patients with prior chemotherapy, corresponding medians were 25.6 months and 26.2 months (HR, 0.91; 95% CI, 0.63-1.32).
Rugo noted that similar findings were seen in patients with endocrine-sensitive disease.
Median OS was prolonged with palbociclib versus placebo for patients who had not received prior chemotherapy for advanced disease at 42.3 and 32.1 months, respectively (HR, 0.68; 95% CI, 0.48-0.96). The absolute difference was 10.2 months. Corresponding rates of median OS were similar in the 2 treatment arms for these patients who had received prior chemotherapy for advanced disease, at 27.6 and 28.0 months (HR, 0.84; 95% CI, 0.54-1.28). Regardless of treatment, median OS was longer in the endocrine- sensitive population than the overall population, Rugo said.
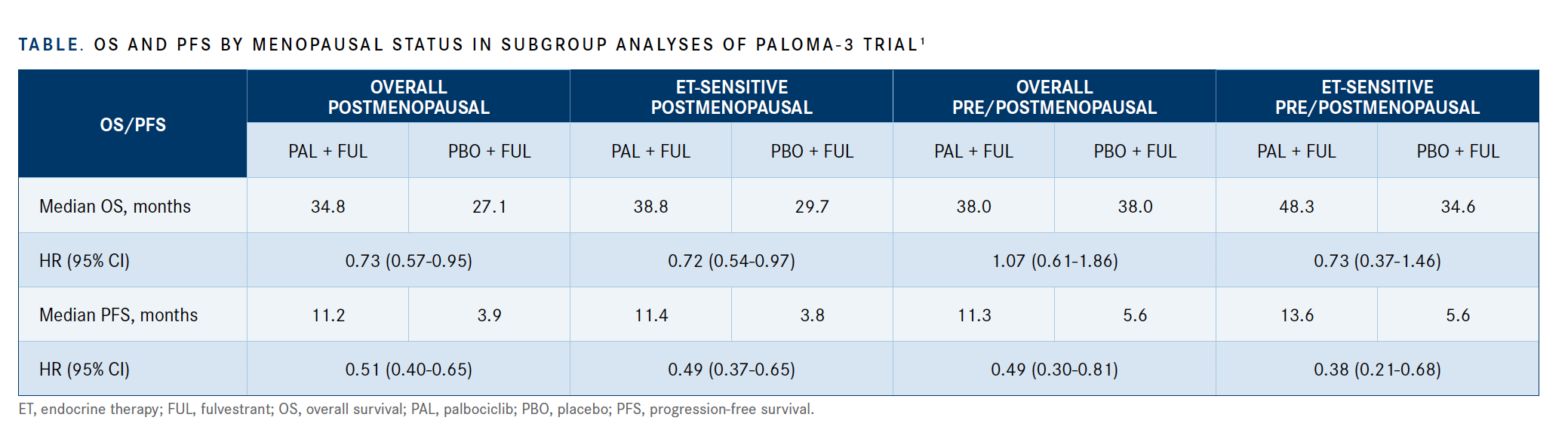
In both the overall and the endocrine-sensitive populations, postmenopausal women treated with palbociclib had a prolonged median OS compared with those receiving placebo in both the overall and the endocrine-sensitive populations (TABLE).1
In patients with visceral and nonvisceral disease, improved OS was observed in patients in the treatment arm compared with the placebo arm, said Rugo. Specifically, among patients with visceral disease in the overall population, those in the palbociclib and fulvestrant arm had a median OS of 27.6 months versus 24.7 months in the placebo arm (HR, 0.85, 95% CI, 0.54-1.13). Among patients with visceral disease who had had no prior chemotherapy and undergone fewer than 2 prior regimens of therapy, those in the treatment arm had a median OS of 28.8 months compared with 24.7 months for patients in the placebo arm (HR, 0.74; 95% CI, 0.47-1.15).
In patients with nonvisceral disease from the overall population, the treatment arm induced a median OS of 46.9 months compared with 35.4 months in patients who received placebo (HR, 0.69; 95% CI, 0.46-1.04). In patients with nonvisceral disease with no prior chemotherapy and fewer than 2 prior regimens, the median OS was 46.9 months for the treatment arm versus 35.4 months for the placebo arm (HR, 0.66; 95% CI, 0.37-1.16).
Unlike the data in postmenopausal women, pre- or perimenopausal women receiving palbociclib in the overall population had a similar median OS compared with those receiving placebo.
“This disparity may be due to the small number of pre- and perimenopausal women compared with postmenopausal women, as well as an increased percentage of premenopausal patients with endocrine resistance, at 30% versus 19%, [versus] postmenopausal women,” Rugo said.
Regarding lines of therapy, those patients receiving placebo had fewer lines of therapy overall: 72% of patients on the placebo arm versus 58% of patients on the palbociclib arm. In the pre- and perimenopausal patients with endocrine-sensitive disease, investigators observed a trend favoring palbociclib versus placebo, with a median OS of 48.3 versus 34.6 months (HR, 0.73; 95% CI, 0.37-1.46).
Improved OS was observed with the addition of palbociclib to fulvestrant in nonvisceral or visceral disease, in patients with no prior chemotherapy for advanced breast cancer, in those with endocrine sensitivity, and in those with fewer prior regimens of treatment.
“These analyses are exploratory, and data must be interpreted with caution, but there may be benefit [to adding palbociclib/fulvestrant before chemotherapy] if patients are treated earlier in the course of the advanced-disease setting,” Rugo concluded.
References:
1. Rugo S, Cristofanilli M, Loibi S, et al. Predictors of efficacy in patients (pts) with hormone receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (HR+/HER2– ABC): subgroup analyses of PALOMA-3. Presented at: 12th European Breast Cancer Conference; September 30-October 3, 2020; virtual. Accessed October 19, 2020. https://bit.ly/3m0USdf
2. Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926-\ 1936. doi:10.1056/NEJMoa1810527