Options Available in Second Line for Patients With TNBC and No Biomarkers
Eight months after completion of adjuvant therapy for triple-negative breast cancer, a 48-year-old patient reported worsening fatigue.
Tiffany A. Traina, MD

During a Targeted Oncology Case-Based Roundtable event, Tiffany A. Traina, MD, an associate attending physician and vice chair of Oncology Care, Department of Medicine, as well as the section head of the Triple Negative Breast Cancer Clinical Research Program at Memorial Sloan Kettering Cancer Center and associate professor of Medicine at Weill Cornell Medicine, discussed a 48-year-old woman with triple-negative breast cancer.
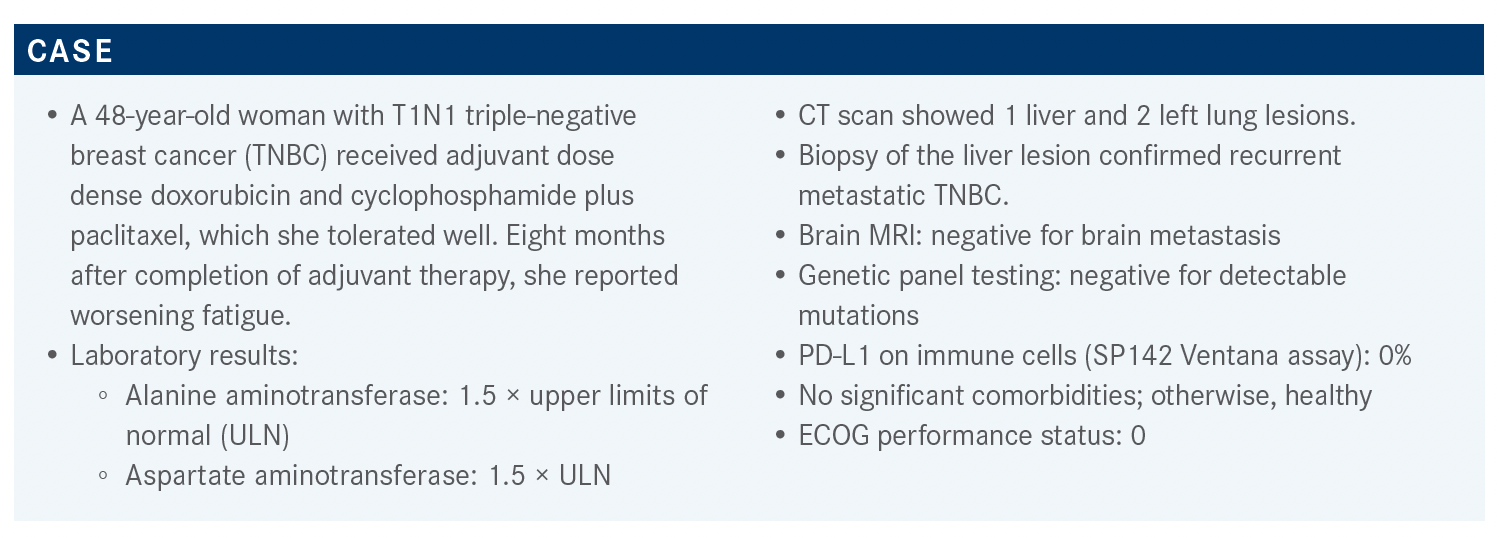
Targeted OncologyTM: Should all women with metastatic TNBC have PD-L1 testing ordered? Which PD-L1 expression assay is used at your institution? Does the assay choice matter?
TRAINA: The IMpassion130 study [NCT02425891] compared first-line albumin-bound (nab)-paclitaxel [Abraxane] plus or minus atezolizumab [Tecentriq]. It was open to all-comer patients with TNBC. They performed PD-L1 testing using SP142 [Ventana PD-L1] and about 40% of the population had positive SP142, which is ultimately the population that’s deriving benefit from atezolizumab. Subsequently, Rugo HS et al1 at the European Society for Medical Oncology Congress 2019 reported concordance with 2 other assays, 22C3 [Dako], which was for the CPS [combined proportion score], or SP263 [Ventana]. The 22C3 had great concordance and about 50% of those with positive SP142 had positive 22C3 as well. However, the 22C3 cutoff in this analysis was 1%, and what we’ve subsequently learned from KEYNOTE-086 [NCT02447003] is that the population that derived the greatest benefit from pembrolizumab [Keytruda] had a CPS of 10% or greater.2 Although these data are available to us, it really doesn’t answer the question about what the concordance between SP142 and a CPS of greater than 10% is, which is what we would see as actionable for pembrolizumab.
We do the SP142 [test] in-house, but there’s also work being done to stand up a CPS assay as well, and we may test both. I have started to see some next-generation sequencing reports coming in that are reporting both antibodies, just until we sort out what the true concordance is.
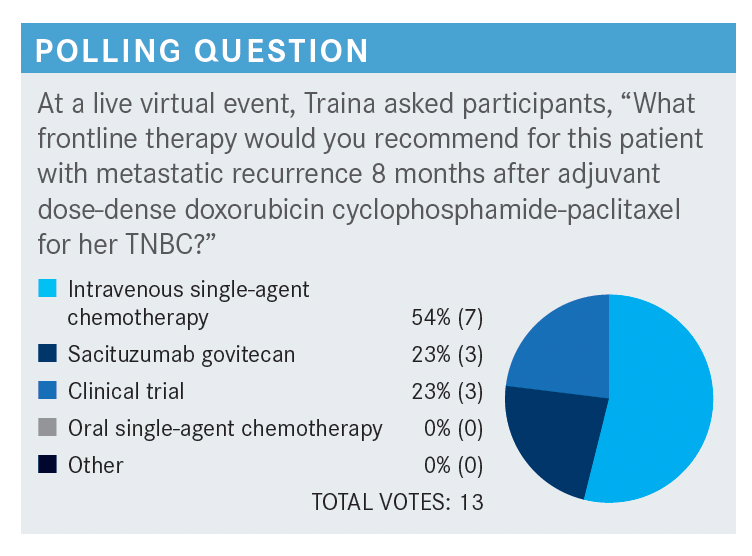
What are some options for this patient? Which factors affect your choices?
She had germline testing and it was wild-type. Certainly platinum [therapy] is reasonable. The NCCN [National Comprehensive Cancer Network] guidelines have carboplatin and cisplatin, specifically, for patients with TNBC or germline mutations in their laundry list of active cytotoxic drugs.3 It’s a very reasonable choice, especially given that she didn’t have it in the early-stage setting and had a very short disease-free interval.
I’m a huge fan of capecitabine [Xeloda], and I think in this case, the thing that just gives me pause is the tempo of the disease in the face of some of our good early-stage chemotherapy agents, the visceral disease as well, and the mildly elevated liver function tests.
It just makes me nervous that maybe capecitabine wouldn’t be adequate, but I have certainly used this also in the setting of first-line [therapy for] TNBC for someone who’s PD-L1 negative but has low-volume disease—maybe bone-only or nodal-only disease and a longer disease-free interval.
Often, I see gemcitabine [Gemzar] getting partnered quite a bit with carboplatin. The KEYNOTE-355 [NCT02819518] regimen for patients with PD-L1–positive [expression] is gemcitabine with carboplatin and pembrolizumab as one of the first-line therapy options.
I think the NCCN guidelines give an out here for doublet chemotherapy in the setting of highly symptomatic, large-burden visceral disease. The NCCN guidelines give a little bit more structure around the laundry list of active cytotoxic agents.
They’re categorized now and call out biomarkers like germline BRCA status because we have good data for use of PARP inhibitors in the setting of germline mutation carriers. They call out platinum agents for TNBC and PD-L1–positive TNBC because of the algorithm down the line of checkpoint inhibitors.3
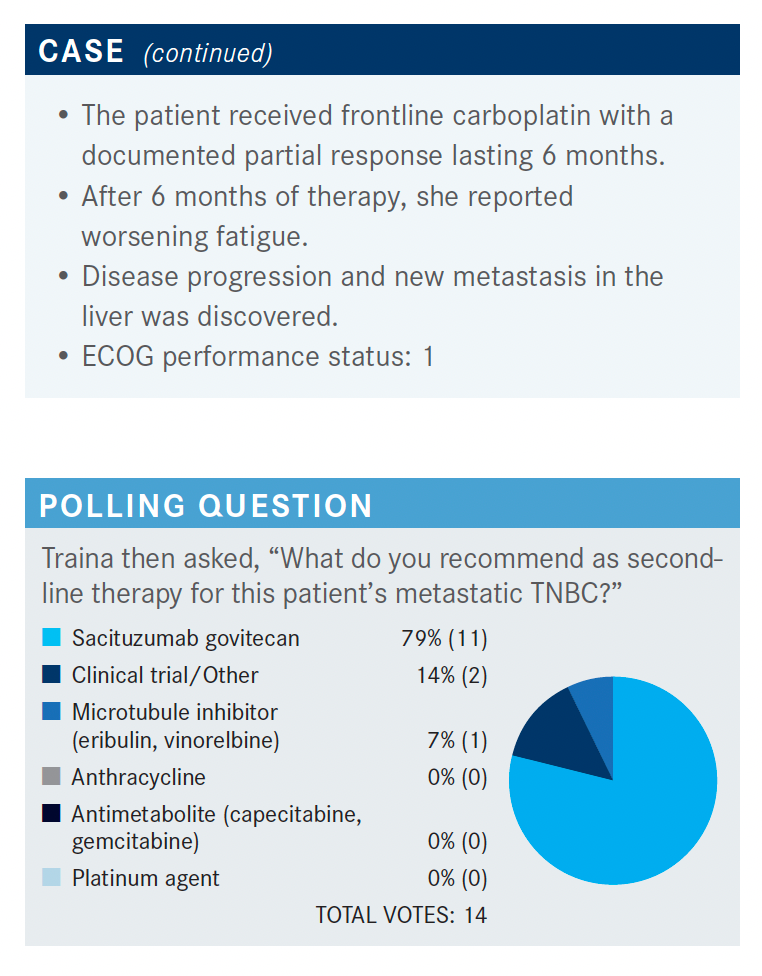
What do you think of these poll responses?
For microtubule inhibitor, often I would reach for eribulin [Halaven]. I would say it became my go-to drug because many of these patients have had anthracycline, taxane, possibly capecitabine, and if they had saw perhaps CR [complete response], they might have seen a platinum in the adjuvant or neoadjuvant setting. So, for the second-line setting, eribulin ended up being my early go-to drug but sacituzumab [Trodelvy] seems to be the choice for most of [the participants].
April 2020 and following the randomized phase 3 ASCENT trial [NCT02574455] results, the FDA granted full approval to sacituzumab in April 2021.4 I thought this was interesting—in the label it calls for an indication in patients with unresectable, locally advanced, or metastatic TNBC who’ve received 2 or more prior therapies, at least 1 being for metastatic disease. I think this is scaling the ASCENT eligibility criteria where patients are now eligible if they’ve had adjuvant chemotherapy and progressed after first-line treatment, so solidly giving that second-line indication; whereas I think the ASCENT trial was a bit strict in its definition of what the disease-free interval was for adjuvant therapy and we thought of it as third-line treatment. This really opens the door for second-line treatment.
What was the makeup of the population on the ASCENT trial?
The eligibility for the ASCENT study was patients who had 2 or more chemotherapy agents for advanced disease. There was no limit to the number of prior therapies; if they recurred within a year of their adjuvant therapy, that counted as 1 of the lines. Sacituzumab was randomized to treatment of physician’s choice [TPC]. It was given 2 weeks on, 1 week off in a 3-week cycle with a starting dose of 10 mg/kg. Patients were treated until progression [of disease or unacceptable toxicity] and the primary end point was progression-free survival [PFS].5
The patient population is what you would expect for TNBC, younger women, 10% of whom were known to have positive germline BRCA, but this was not routinely done in all patients. Most of these patients had TNBC in the adjuvant setting and then had recurred, and the time to metastatic disease was rather quick, just over a year, before developing metastatic breast cancer.
Many of these patients had visceral metastases and multiple prior lines of therapy, so the median prior lines of therapy was 3 with the range going up to 16 prior lines of chemotherapy, which is impressive. The drugs used were our typical go-to active agents in breast cancer. The proportion of patients who had prior PARP inhibitors was on track with those who were known to be germline mutation carriers, and about one-third of these patients had prior checkpoint inhibitors.
The primary end point of PFS [in the population without brain metastases] was significant with a 60% improvement in PFS with sacituzumab compared with TPC. I think one of the really sobering takeaways is how poorly these patients did with standard-of-care chemotherapy. The median PFS with TPC was under 2 months and with sacituzumab it was almost 6 months [HR, 0.41; 95% CI, 0.32-0.52; P < .0001]. The overall survival [OS] was just incredibly meaningful with more than a 50% improvement in median OS, and again, the control arm does terribly with only a median OS of 7 months, a near doubling of median OS with sacituzumab over TPC [HR, 0.48; 95% CI, 0.38-0.59; P < .0001].
In the forest plot for the PFS primary end point, nearly all groups favored the antibody-drug conjugate sacituzumab over chemotherapy. The Asian population had a wide confidence interval range [HR, 0.40; 95% CI, 0.08- 2.08], but it was a total of only 18 patients, so I would not overread that. All groups appeared to be deriving benefit.
The sacituzumab waterfall plot showed many patients had a RECIST response and derived the clinical benefit of stable disease of more than 6 months compared with TPC. Time to response was 1.5 months, so when you’re thinking about symptomatic relief maybe by the second cycle, patients are really having response and benefit. The duration of response was twice as long with sacituzumab [at 6 months] compared with TPC [which was 3 months].
The primary end point of PFS in the full population including those who had brain metastases showed about 60% improvement in PFS with sacituzumab compared with TPC. The median PFS was 4.8 months with sacituzumab compared with 1.7 months with TPC chemotherapy [HR, 0.43; 95% CI, 0.35-0.54; P < .001].
The main things that rose to the top when looking at grade 3 or 4 toxicity for sacituzumab were cytopenias, specifically neutropenia, as well as gastrointestinal toxicity, which was at about 10% grade 3 diarrhea. These are things that you can do prophylaxis against, anticipate, educate, and rely heavily on nurses and supportive care to really help mitigate. In comparison, to say irinotecan, because the payload on this antibody-drug conjugate is the metabolite of irinotecan, what we’re seeing here in grade 3 gastrointestinal toxicity is much improved.
What therapies did patients randomized to the comparator arm receive? How does that inform how you use sacituzumab govitecan vs other options?
More than 50% of the patients were getting eribulin in the second-line or third-line setting. Pretty much all the other standard cytotoxic chemotherapy agents were all overlapping with poor median PFS. The median OS showed gemcitabine [had the closest OS to sacituzumab at 8.4 months compared with 12.1 months for sacituzumab], but the smallest number of patients [12%] had gotten gemcitabine.6 It’s nice to have the subset analysis but remember that the overall analysis was TPC as a pooled end point vs sacituzumab.
Have we learned anything about the value of potential biomarkers to guide use of this agent (eg, Trop-2 expression, germline BRCA1 or BRCA2 mutation status)?
They looked at BRCA status and the subset was about 34 patients known to be BRCA1 or BRCA2 positive vs the remainder of the population that was known to be negative. Sacituzumab had activity both in patients with the germline mutations as well as those in the wild-type arm. Interestingly, the mutation carriers had a lower response rate at 19% vs 33% for sacituzumab in the wild-type arm. But these patients were heavily pretreated [and that is not factored in.] I think it’s enough to say that the drug had activity regardless of BRCA status.
The Trop-2 expression was also looked at as a correlative study here. A majority of patients—80%—had medium to high Trop-2 expression, and even some of those patients with low Trop-2 expression had potentially some benefit from sacituzumab, so there is no requirement for Trop-2 testing as a predictive biomarker for benefit, which keeps it simple for patients.7 I think one of the most frustrating experiences in caring for a woman with TNBC is when you hunt for all these different potential biomarkers and they’re all negative, leaving us with the option of cytotoxic chemotherapy. It is nice to know we don’t need a biomarker to see benefit.
For the age cutoff, a [subset analysis] data piece from the American Society of Clinical Oncology looked at efficacy in those under vs over age 65 years.8 The benefit was maintained regardless of age, but for toxicity, probably not surprisingly, the population that was 65 years or older had more frequent adverse events [AEs] and dose reductions, which was true of the control arm too. I think we just need to be aware of that and cautious. Treatment discontinuation due to AEs was the same in both age groups, at 2%.
Remembering our goal is palliative therapy, it is encouraging to know efficacy is there, toxicity is manageable, and although the number of patients over age 75 years was small, the rates of AEs did not appear to be any greater than in the population that was between 65 and 75 years old.8
REFERENCES:
1. Rugo HS, Loi S, Adams S, et al. 6571 - Performance of PD-L1 immunohistochemistry (IHC) assays in unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC): post-hoc analysis of IMpassion130. Ann Oncol. 2019;30(suppl 5):v851-v934. doi:10.1093/annonc/mdz394
2. Adams S, Schmid P, Rugo HS, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):397-404. doi:10.1093/annonc/mdy517
3. NCCN. Clinical Practice Guidelines in Oncology. Breast cancer, version 8.2021. Accessed September 29, 2021. https://bit.ly/3kxcYWm
4. FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer. FDA. Updated April 8, 2021. Accessed September 29, 2021. https://bit.ly/3ARnLkd
5. Bardia A, Hurvitz SA, Tolaney SM, et al; ASCENT Clinical Trial Investigators. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529-1541. doi:10.1056/NEJMoa2028485
6. O’Shaughnessy J, Punie K, Oliveira M, et al. Assessment of sacituzumab govitecan (SG) versus treatment of physician’s choice (TPC) cohort by agent in the phase 3 ASCENT study of patients (pts) with metastatic triple-negative breast cancer (mTNBC). J Clin Oncol. 2021;39(suppl 15):1077. doi:10.1200/JCO.2021.39.15_suppl.1077
Bardia A, Tolaney SM, Punie K, et al. Biomarker analyses in the phase III ASCENT study of sacituzumab govitecan versus chemotherapy in patients with metastatic triple-negative breast cancer. Ann Oncol. 2021;32(9):1148-1156. doi:10.1016/j.annonc.2021.06.002
7. Kalinsky K, Oliveira M, Traina TA, et al. Outcomes in patients (pts) aged ≥65 years in the phase 3 ASCENT study of sacituzumab govitecan (SG) in metastatic triple-negative breast cancer (mTNBC). J Clin Oncol. 2021;39(suppl 15):1011. doi:10.1200/JCO.2021.39.15_suppl.1011