Novel Immunotherapy for Pancreatic Cancer May Allow for Resection of the Unresectable
The phase III PILLAR trial studying the novel immunotherapy, algenpantucel-L combined with chemotherapy in patients with locally advanced, unresectable pancreatic cancer, has completed enrollment with over 300 patients.
Novel Immunotherapy for Pancreatic Cancer May Allow for Resection

Harish Lavu, MD
The phase III PILLAR trial, which is studying the novel immunotherapy algenpantucel-L combined with chemotherapy in patients with locally advanced, unresectable pancreatic cancer, has completed its enrollment with over 300 patients, according to a press release1from the manufacturer, NewLink Genetics Corporation.
“Obtaining a treatment response with conventional therapies has been difficult. The hope is that combining these therapies with algenpantucel-L immunotherapy will result in an increased rate of tumor down-staging such that we can get more patients to surgical resection, which is the most effective treatment for pancreatic cancer,” said Harish Lavu, MD, associate professor of surgery, Thomas Jefferson University, and lead investigator for PILLAR, in an interview withTargeted Oncology.
The PILLAR study2will randomize patients with borderline resectable or locally advanced, unresectable pancreatic cancer who have an ECOG performance status of ≤1, to either an algenpantucel-L plus chemotherapy arm or chemotherapy alone arm. Patients receiving algenpantucel-L will be given 300 million cells by interdermal injection on days 1 and 8, followed up by doses every 2 weeks for up to 18 doses. These will be combined with FOLFIRINOX administered on days 22, 36, 50, and 64. The chemotherapy-alone arm will receive FOLFIRINOX on days 1, 15, 29, 43, and 57.
Patients with active metastases were disqualified for the study, as were those receiving immunosuppressive therapy or chronic systemic corticosteroid therapy. The study's primary endpoint is overall survival (OS) with a secondary endpoint being progression-free survival. Frequency and grade of adverse events (AEs) of the combination therapy will also be observed. The estimated study completion date is June 2017.
Algenpantucel-L, is also being studied in the larger phase III IMPRESS trial. This trial includes 722 patients with surgically resected pancreatic cancer.3The phase III IMPRESS trial followed positive results from the IMPRESS phase II trial, where patients administered algenpantucel-L plus standard of care therapy experienced an 81% disease-free survival (DFS) and an OS of 96%.1
The phase II multicenter, open label trial4evaluated algenpantucel-L plus chemotherapy in 69 patients with resected pancreatic cancer in the adjuvant setting. Patients received either 100 million cells/dose or 300 million cells/dose given intradermally with gemcitabine 1000 mg/m2and chemoradiotherapy + 5-FU 250 mg/m2, within 6 weeks following surgery. Patients completing therapy received up to 14 doses of algenpantucel-L. The primary objective was DFS defined as the percentage of patients with no disease recurrence 12 months following surgery. Secondary objectives included OS and toxicity. Grade 3 AEs were <12% and included fatigue, injection site reaction, pain, and lymphopenia.
Algenpantucel-L has received fast-track status and orphan drug designation from the FDA. NewLink Genetics and the FDA agreed in January 2010 on a Special Protocol Assessment regarding the trial design, clinical endpoints, and a statistical analyses plan for IMPRESS to be used in support of a Biologic License Application (BLA).3
According to the NewLink Genetics’ website,4algenpantucel-L consists of two altered pancreatic cancer cell lines (HAPa-1 and HAPa-2) modified to express galactose-alpha-1,3-galactose, or alpha-gal, a mammalian oligosaccharide epitope found in most organisms’ cell membranes, but not in primates.5
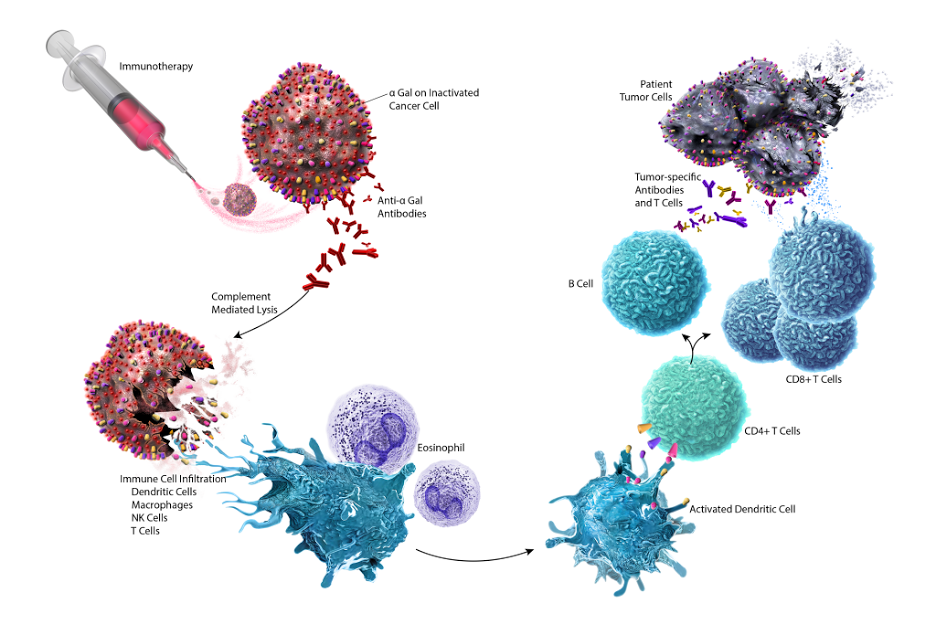
Proposed mechanism of action for algenpantucel-L. Image courtesy of NewLink Genetic Corporation.
The premise behind the mechanism of action for algenpantucel-L is that pre-existing anti-alpha-gal antibodies target the drug in vivo. Algenpantucel-L cell debris may then be taken up and processed by the immune system. Cancer-specific antigens resulting from this process may then be shared by cancer cells in the patient, which allows the immune system to recognize and destroy the cancer.4
“The drug works to train the patient’s immune system to recognize their pancreatic cancer as being foreign and to mount an immune response against it,” Lavu said. “There is an unmet need among patients with locally advanced as well as resectable pancreatic cancer. This trial is designed to test whether or not algenpantucel-L is effective in improving outcomes among these patients.”
References
- NewLink Genetics. December 15, 2015. NewLink Genetics Corporation completes enrollment of phase 3 PILLAR trial evaluating algenpantucel-L for patients with locally advanced pancreatic cancer.http://investors.linkp.com/releasedetail.cfm?ReleaseID=946998. Accessed December 15, 2015.
- ClinicalTrials.gov Identifier: NCT01836432.https://clinicaltrials.gov/ct2/show/study/NCT01836432?view=record. Accessed December 15, 2015.
- Nasdaq GlobeNewswire. NewLink Genetics IMPRESS phase 3 pancreatic cancer trial with algenpantucel-L to continue following second interim analysis.http://globenewswire.com/news-release/2015/05/11/734701/10133840/en/NewLink-Genetics-IMPRESS-Phase-3-Pancreatic-Cancer-Trial-with-Algenpantucel-L-to-Continue-Following-Second-Interim-Analysis.html. Accessed December 16, 2015.
- NewLink Genetics. Pancreatic cancer.http://www.newlinkgenetics.com/platforms/hyperacute-immunotherapies/pancreatic-cancer/. Accessed December 15, 2015.
- Commins SP and Platts-Mills TA. Delayed anaphylaxis to red meat in patients with IgE specific for galactose alpha-1,3-galactose (alpha-gal).Curr Allergy Asthma Rep. 2013 Feb;13(1):72-7. doi: 10.1007/s11882-012-0315-y.