Multimodal Therapy Explored in Unresectable HCC
Findings from the use of mutimodal interventional therapy of lenvatinib and transarterial therapy may lay the groundwork for future research regarding the combination for the treatment of patients with hepatocellular carcinoma.
Wei Lu, MD, PhD

The mutimodal interventional therapy of lenvatinib (Lenvima), PD-1 inhibition, and transarterial therapy was safe and effective in patients with unresectable hepatocellular carcinoma (HCC), according to findings from a propensity score-match analysis presented in a poster at the European Association for the Study of the Liver’s 2022 Liver Cancer Summit.
“Transarterial chemoembolization [TACE] and hepatic arterial infusion chemotherapy [HAIC] are also recommended treatments for patients with intermediate to advanced HCC. Because HCC is a tumor with rich blood supply, TACE or HAIC can improve the concentration of chemotherapy drugs in the tumor, which can improve the curative effect on the one hand and reduce the systemic adverse events on the other hand. Clinical studies have confirmed that the ORR [overall response rate] of patients with intermediate HCC can be more than 50% with TACE alone,” said lead author Wei Lu, MD, PhD, of the Tianjin Medical Univer- sity Cancer Institute and Hospital in China, in an interview with Targeted Therapies in OncologyTM
The investigators conducted a retrospective study analyzing 70 patients with intermediate or advanced HCC who underwent therapy with len- vatinib, PD-1 inhibition, and transarterial therapy (LEN-PD1-TH) and compared outcomes with patients who only received lenvatinib and anti–PD-1therapy(LEN-PD1).Patientsweretreated at Tianjin Cancer Hospital between December 2018 and October 2020. The study’s primary end point was progression-free survival (PFS), with the secondary end points being overall survival (OS) and ORR.
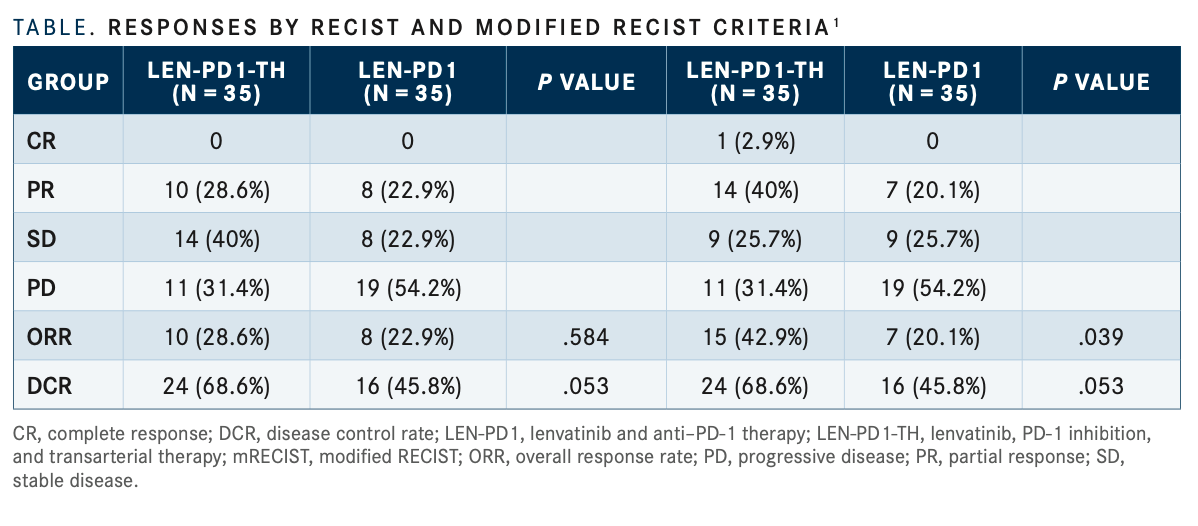
At a median follow-up of 14 months (95% CI, 10.1-17.9), the LEN-PD1-TH group achieved a longer median PFS compared with the LEN-PD1 group, 15 months vs 9 months, respectively (P =.004). OS was also improved, as the median OS was not reached in the LEN-PD1-TH cohort and was 14 months in the LEN-PD1 cohort (P <.001).
ORR, as determined by modified RECIST criteris was significantly higher in the LEN-PD-1-TH group at 42.9% compared with the LEN-PD1 group at 20.1% (P =.039). One patient in the LEN-PD1-TH group achieved a complete response and 14 patients had partial responses vs 7 with partial responses in the LEN-PD1 group; 9 patients had stable dis- ease in each group. By regular RECIST criteria, the ORRs were 28.6% and 22.9% in the LEN-PD1-TH and LEN-PD groups, respectively (TABLE1).
This study is the first to report that the efficacy of lenvatinib combined with PD-1 inhibitors and interventional therapy is better than that of lenvatinib combined with PD-1 inhibitors when the baseline level is balanced and the number of [participants] in the 2 groups is the same. Based on this, we hope our results can provide a theo- retical basis or enlightenment for future related research,” the authors wrote in the poster.
Subgroup analyses of the findings also showed that patients younger than 65 years who had liver cirrhosis, Child-Pugh class A, extrahepatic metastasis, no vascular invasion, tumors smaller than 10 cm and more than 3 tumors, Barcelona Clinic liver cancer stage C, and α-fetoprotein at or under 400 ng/mL were more likely to benefit from the 3-drug combination in terms of both PFS and OS. For all other patients, the LEN-PD1-TH and LEN-PD1 groups had comparable prognostic efficacy.
There were 70 patients included in the safety analysis, and the most common grade 3 or higher adverse event was hypertension in both treat- ment groups (17.1% and 20.0% in the LEN-PD1 and LEN-PD1-TH cohorts, respectively).
Because the most common adverse reaction of the combined regimen is hypertension, patients should pay attention to monitoring blood pressure and take oral antihyperten- sive drugs in time if blood pressure rises. In addition, timely review of blood routine, liver function, and other indicators can timely formulate treatment plans according to their own adverse reactions,” the authors wrote.
Investigators observed 2 grade 3 hand- foot-skin reactions and 1 grade 3 bleeding event in the LEN-PD1 group. In the LEN-PD1- TH group, there was 1 grade 3 decreased platelet count and 1 grade 3 muscle pain event. Ultimately, the investigators noted that there was not a significant difference in adverse events between the 2 groups.
Of note, patients enrolled in the study were prescribed 8 mg of daily lenvatinib instead of the standard dose of 12 mg to make the therapy more tolerable and ensure that they would complete as many treatment courses as possible.
Ultimately, the investigators are hoping that their findings lay the groundwork for future research regarding the combination use of lenvatinib, PD-1 therapy, and transarterial therapy.
“The next step is to continue to collect patients, expand cases, and continue follow-up. If possible, we intend to conduct prospective research to further verify our conclusions,” they said.
REFERENCE:
Lu W, Lang M, Song T, et al. Efficacy of combination therapy with lenvatinib, programmed cell death 1 inhibitors and transarterial therapy: a propensity score-matching cohort study. Presented at: EASL Liver Cancer Summit 2022; February 3-4, 2022; virtual. Ab- stract PO-119.
Survivorship Care Promotes Evidence-Based Approaches for Quality of Life and Beyond
March 21st 2025Frank J. Penedo, PhD, explains the challenges of survivorship care for patients with cancer and how he implements programs to support patients’ emotional, physical, and practical needs.
Read More