Metastatic Colorectal Cancer Target May Prove Promising for Subset of Patients
Recent results from a survey of some 7000 patient specimens from the Cancer Genome Atlas concluded that gene fusion events, resulting in oncogenic activation, were commonly observed among members of the neurotrophic tyrosine receptor kinase family, with 23 gene fusions observed over a total of 9 different tumor types, including colorectal cancer.
Recent results from a survey of some 7000 patient specimens from the Cancer Genome Atlas concluded that gene fusion events, resulting in oncogenic activation, were commonly observed among members of the neurotrophic tyrosine receptor kinase (NTRK) family, with 23 gene fusions observed over a total of 9 different tumor types, including colorectal cancer.1These results and others have implicated members of the Trk familyTrkA, TrkB, and TrkC—encoded by the genesNTRK1,NTRK2,andNTRK3, respectively, as being potentially important oncogenic drivers for a subset of patients with metastatic colorectal cancer (mCRC).1With increasing recognition of these rare molecular alterations, the stage is set to identify mCRC patients who might benefit from targeted therapy with pan-Trk inhibitors that are now being actively evaluated in the clinic. A new phase II global trial of entrectinib, a potentTrk inhibitor, will add additional clinical interest beyond typicalcolorectal cancer clinical trialsin these genes.
Molecular Alterations inNTRKin Metastatic Colorectal Cancer (mCRC)
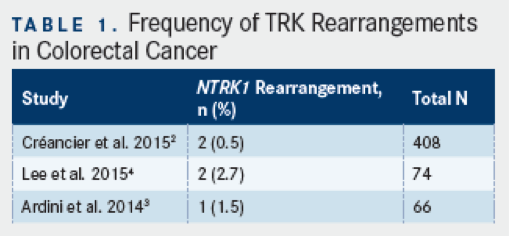
Using immunohistochemistry (IHC) and reverse transcriptase (RT)-PCR methods, a recent survey of 408 colorectal cancer specimens found a total of two rearrangements inNTRK1(0.5%), one corresponding to a previously described fusion between tropomyosin 3 (TPM3)andNTRK1, and a second, newly observed rearrangement in colon cancer, translocated promoter region(TPR)-NTRK1.2TheTPM3-NTRK1fusion, as further described below, has been estimated to occur in about 1% of colon cancers, and in preclinical models, this gene fusion has been shown to encode a constitutively activated kinase that is hypersensitive to Trk inhibition.3In another study that evaluated 74 CRC specimens,NTRK1gene rearrangements were detected in two cases (2.7%), both of which corresponded to theTPM3-NTRK1fusion.4
Asked about the overall frequency ofTRKmutations, Marwan Fakih, MD, professor of Medical Oncology and Experimental Therapeutics, and Section Head of GI Medical Oncology at City of Hope Comprehensive Cancer Center, Duarate, CA, noted that, at present, there is fairly limited information regarding the frequency ofNTRKrearrangementsin mCRC. “Studies on colorectal cancer specimens have reported an incidence rate ranging from 0.5% to 2.7%” he said, referencing the above data. Current information aboutNTRKin mCRC has generally been limited to date, due to an infrequency of molecular testing. “Unfortunately, testing for gene fusion alterations is not done routinely for patients with metastatic colorectal cancer, therefore limiting the ability to identify patients with such alterations,” Fakih said.
mCRCNTRKRearrangements: Focus onTPM3-NTRK1
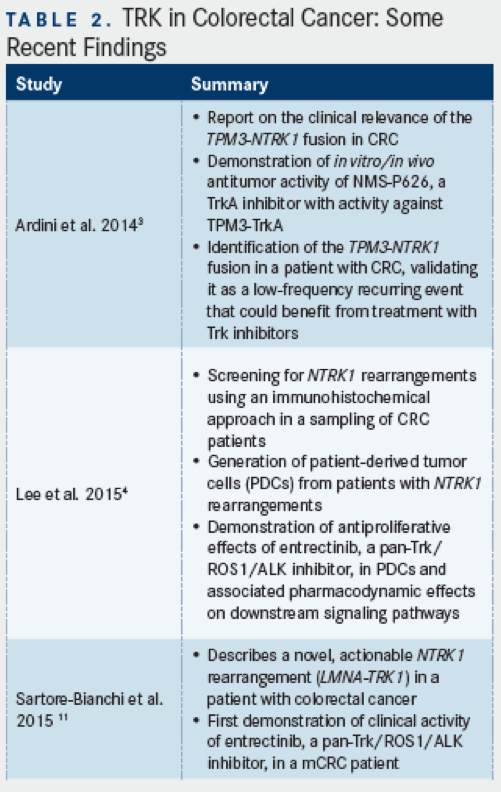
TPM3-NTRK1was first identified as a transforming gene rearrangement in colon carcinomas nearly 30 years ago, but only more recently has a 2-step analytic approach been developed and validated for the purpose of detecting tumors withNTRK1rearrangements.3,5This assay was applied to a sampling of 66 formalin-fixed, paraffin-embedded (FFPE) patient surgical specimens, and one sample (1.5%) was identified and shown to harbor theTPM3-NTRK1gene rearrangement, as detected by IHC with antibody specific for TrkA, but not other TRK family members (ie, TrkB or TrkC) and reverse transcription quantitative PCR (using primers to span theTPM3-NTRK1rearrangement junction). This study also showed that the same TPM3-TrkA fusion protein was present in KM12 cells, which showedin vitrosensitivity to a small-molecule inhibitor of TrkA, termed NMS-P626 (a molecular precursor to entrectinib, theNTRKinhibitorin the latest stage of clinical development); notably, this and other compounds with inhibitory activity for TrkA at <50 nM were found to have potent inhibitory activity for TrkA at <50 nM were found to have potent inhibitory activity on KM12 cell proliferation (IC50<500nM), suggesting a critical role for this kinase in the cell line.3
These investigators further characterized NMS-P626, a pyrazolo-pyridine molecule having a favorable pharmacokinetic profile (ie, good steady state volume of distribution, high oral bioavailability, and low plasma clearance), and inhibitory activity for TrkA, as well as the related TrkB, and TrkC kinases (IC50values of 8nM, 7nM, and 3nM, respectively). The antiproliferative activity of this molecule for KM12 cells (IC5019nM), which were shown to express the TPM3-TrkA fusion, was remarkably specific, relative to some 78 other cell lines examined from diverse tissue types.3TrkA immunoreactivity in KM12 cells was found to be cytoplasmic, as opposed to being localized at the cell surface, as would be expected with the normal TrkA receptor.
Moreover, the TPM3-TrkA fusion protein was found to be in a highly phosphorylated, activated state in untreated KM12 cells, and upon treatment with NMS-P626, autophosphorylation of the fusion protein could be almost completely suppressed, as could the activation of downstream signaling/effector molecules including PLCg, ALK, and ERK.3Accumulation of cells in G1 phase, and an increase in the proportion of cells undergoing apoptosis, was also observed with NMS-P626 treatment. Lastly,in vivoantitumor activity experiments in nude mice with established KM12 tumors showed over 90% tumor growth inhibition with orally administered NMS-P626 at 50 or 100 mg/kg (twice daily for 10 consecutive days), relative to vehicle-treated animals, and these effects were observed without discernable weight loss or toxicity.
Other TRK Family Members in CRC
Published evidence has also suggested that expression of TrkB, a product of theNTRK2gene, and receptor for the brain-derived neurotrophic factor (BDNF) ligand, may have some biologic and/or prognostic relevance in CRC. Earlier work had shown that the BDNF/TrkB axis was associated with suppressing anoikis, or apoptosis in response to loss of cell-matrix interaction.6Tanaka and coworkers used IHC to examine BDNF and TrkB expression in a sample of 223 CRC patients; they found that patients with higher BDNF, TrkB, and co-expression of BDNF and TrkB had poor prognosis, and co-expression was further associated with liver and peritoneal metastasis.7They also found that,in vitro, BDNF expression could increase cell viability, invasive and migratory capability, and suppress anoikis in TrkB-expressing cell lines, and these effects could be abrogated with the use of a pan-Trk inhibitor (K252a).7Earlierin vitrowork from De Farias and colleagues had shown that the same BDNF/TrkB axis was associated with resistance to agents targeting the epidermal growth factor receptor (EGFR), such as cetuximab, in CRC cells, and that blockade with K252a could overcome this potential resistance pathway.8
Sasahira and co-workers also used IHC analysis to evaluate TrkB and TrkC expression in a sample of 133 CRC patients. They found positive expression of TrkB and TrkC by IHC in 23.3% and 12.8%, respectively, in this series.9In addition, TrkB expression was significantly associated with local progression, lymph node, and peritoneal metastasis, whereas TrkC expression was associated only with liver metastasis.9In vitroresults in CRC cell lines further showed that TrkB increased cell growth, while TrkC increased invasive capacity, and both receptors showed anti-apoptotic effects. Collectively, although the role of other Trk family members as well as their degree of overexpression and/or mutation in CRC patients remains to be further elucidated, these findings support possible additional benefits of pan-Trk inhibition as a target in CRC, perhaps in addition to their specific use in patients with Trk rearrangements.
Clinical Inhibition of TRK Rearrangements: Entrectinib
Using patient-derived cells from a metastatic lymph node of a mCRC patient shown to harbor the TPM3-TrkA fusion protein by IHC, Lee and coworkers recently demonstrated that these cells were negative forROS1orALKrearrangement or protein expression, but displayed sensitivity to entrectinib, an inhibtor of TrkA, B, and C, as well as ROS1 and ALK.4TrkA phosphorylation and downstream signaling pathways were also potently inhibited by entrectinib in these patient-derived cells. The results of this study have thus implicatedNTRKrearrangements as a potential target for mCRC and demonstrated the potential utility of Trk inhibition with entrectinib in patients found to have such rearrangements. A late-breaking abstract presented at this year’s European Society for Medical Oncology (ESMO) meeting reported a 75% response rate with entrectinib seen among 4 patients withNTRKgene rearrangements who were treated at or above the recommended phase II dose. Overall the clinical update detailed an objective response rate of 72% across 18 patients with solid tumors harboring gene rerrangements ofNTRK1/2/3,ROS1, orALKwho had not received prior ROS1 or ALK inhibitor therapy and who were treated at or above the RP2D.10The favorable results seen in this population of patients with relevant molecular alterations (includingNTRKalterations) have led to the initiation of a potentially registration-enabling global phase II basket study (STARTRK-2 trial) that began in late 2015.
A recent report published in theJournal of the National Cancer Instituteauthored by Sartore-Bianchi and colleagues has also presented evidence for a new chromosome 1 gene rearrangement involvingNTRK1in a patient with mCRC.11They describe the case of an elderly woman with colon adenocarcinoma with hepatic and adrenal metastatic disease, progressing without response to previous therapies; molecular testing of this patient revealed wild-typeRASandBRAF, but aberrantly high expression of TrkA in the primary tumor and liver metastasis as detected by IHC. The FISH pattern, however, appeared to differ from the previously describedTPM3-NTRK1fusion in mCRC, and as such, these investigators identified a novel fusion of the Lamin A(LMNA)gene withNTRK1resulting in the expression of 2 aberrant splice variant mRNAs and 2 highly phosphorylated fusion proteins (indicative of constitutively activated TrkA kinase).11Accordingly, downstream signaling molecules from TrkA also appeared to be activated. These data prompted treatment of the patient with entrectinib (1600 mg/m2) on a four-day on, three-day off schedule for 3 consecutive weeks every 28 days. Response assessment was first attempted by CT after 4 weeks of treatment, and showed partial response, with 30% reduction in the patient’s target lesions.11Four weeks thereafter, response was confirmed by CT, with marked reduction in hepatic lesions, as well as reduction in the patient’s adrenal gland and peritoneal lesions.
Looking Forward
Fakih was encouraged by these recent clinical findings, although he notes that the dosing regimen in the Sartore-Bianchi report may have limited the durability of response for this one patient. “This case is a proof-of-principle report on the importance ofNTRKrearrangements as a carcinogenesis driver and validatesNTRKas a therapeutic target in patients with colorectal cancer. The response in this patient was not durable, lasting only 4 months. The lack of durability may be attributed to the intermittent dosing of entrectinib this patient received. More protracted responses were noted in other tumors withNTRKrearrangements, treated with continuous entrectinib dosing. The ongoing clinical trial evaluating entrectinib in colorectal cancer withNTRKrearrangements is using a continuous daily dosing schedule.”10Beyond this first clinical report, Fakih also emphasized the evidence for an impact of Trk inhibition in patient-derived xenograft (PDX) models. “Studies on colorectal PDX harboringNTRKrearrangements have shown sensitivity to the Trk inhibitor entrectinib. These results suggest a clinical role for this pan-inhibitor of Trk, ROS1, and ALK, [entrectinib], and support the investigation of this agent in a cohort of colorectal cancer [patients] withNTRK1-3rearrangements, in an ongoing basket clinical trial.”10
Fakih also sees the possibility for expanding molecular testing and the subsequent identification of patients withNTRKrearrangements in mCRC with ongoing studies like the entrectinib basket trial. “This study will provide a treatment option for patients with mCRC withNTRK,ROS1,andALKrearrangements. If the study confirms the findings seen on the phase I clinical trial, this study may pave the way to a path for routine screening (and treatment) forNTRKrearrangements in patients with metastatic colorectal cancer.”
For more information on the phase II trial for entrectinib in patients withNTRK,ROS1, andALKgene rearrangements, please visitwww.STARTRKtrials.com.
References
- Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer.Nat Commun.2014; 5:4846.
- Créancier L, Vandenberghe I, Gomes B, et al. Chromosomal rearrangements involving the NTRK1 gene in colorectal carcinoma.Cancer Lett.2015; 365(1):107-111.
- Ardini E, Bosotti R, Borgia AL, et al. The TPM3-NTRK1 rearrangement is a recurring event in colorectal carcinoma and is associated with tumor sensitivity to TRKA kinase inhibition.Mol Oncol.2014; 8(8):1495-1507.
- Lee SJ, Li GG, Kim ST, et al. NTRK1 rearrangement in colorectal cancer patients: evidence for actionable target using patient-derived tumor cell line.Oncotarget.2015. doi: 10.18632/oncotarget.5494. [Epub ahead of print]
- Martin-Zanca D, Hughes SH, Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences.Nature.1986; 319(6056): 743-748.
- Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB.Nature.2004;430(7003):1034-1039.
- Tanaka K, Okugawa Y, Toiyama Y, et al. Brain-derived neurotrophic factor (BDNF)-induced tropomyosin-related kinase B (Trk B) signaling is a potential therapeutic target for peritoneal carcinomatosis arising from colorectal cancer.PLoS One.2014; 9(5):e96410. doi: 10.1371/journal.pone.0096410.
- de Farias CB, Heinen TE, dos Santos RP, Abujamra AL, Schwartsmann G, Roesler R. BDNF/TrkB signaling protects HT-29 human colon cancer cells from EGFR inhibition.Biochem Biophys Res Commun.2012; 425(2): 328-332.
- Sasahira T, Ueda N, Kurihara M, et al. Tropomyosin receptor kinases B and C are tumor progressive and metastatic marker in colorectal carcinoma.Hum Pathol.2013; 44(6):1098-1106.
- Siena S. Entrectinib (RXDX-101), an oral pan-Trk, ROS1, and ALK inhibitor in patients with advanced solid tumors harboring gene rearrangements. Poster presented at: European Society for Medical Oncology (ESMO) Meeting; September 27, 2015: Vienna, Italy. Late Breaking Abstract (LBA29).
- Sartore-Bianchi A, Ardini E, Bosotti R, et al. Sensitivity to entrectinib associated with a novel LMNA-NTRK1 gene fusion in metastatic colorectal cancer.J Natl Cancer Inst.2015; 108(1). pii: djv306. doi: 10.1093/jnci/djv306.
Taken together, the low frequency ofTPM3-NTRK1gene rearrangements supports its clinical relevance for a subset of CRC patients who may be candidates for emerging Trk-specific therapies such as entrectinib (see below).3The authors also suggest that the IHC screening approach developed in this study may facilitate more rapid screening in larger numbers of patient samples, as opposed to more costly and time-consuming methods such as fluorescence in situ hybridization (FISH).3