Interpreting Trial Data on Tolerability in Third-Line mRCC Regimens
During a Targeted Oncology™ Case-Based Roundtable™ event, Pedro C. Barata, MD, MSc, and participants discussed dosing and toxicity issues related to tivozanib and lenvatinib/everolimus in a patient who has received 2 prior lines of therapy for metastatic clear cell renal cell carcinoma.
Pedro C. Barata, MD, MSc
MODERATOR
Director, Clinical Genitourinary
Medical Oncology Research Program
UH Seidman Cancer Center
Associate Professor of Medicine
Case Western Reserve University
Cleveland, OH

EVENT REGION Pennsylvania and West Virginia
PARTICIPANT LIST Hayman Salib, MD | Pritish Iyer, MD | Monica Malhotra, MD | Mariya Apostolova, MD | Nirmala Nathan, MD
CASE SUMMARY
A 71-year-old man with a history of metastatic renal cell carcinoma (mRCC) had left nephrectomy and adrenalectomy. Four years later, he experienced disease recurrence with lung nodules and biopsy results consistent with clear cell RCC. It was subsequently recognized that nodules had been present on scans from 2 prior years. He was observed based on low volume, indolence of disease, and patient preference.
Eighteen months later, scans showed continued indolent growth, increased total tumor burden, a new paratracheal lymph node (20 × 15 mm), and multiple growing pulmonary nodules. A decision was made to initiate systemic therapy with pembrolizumab (Keytruda) plus axitinib (Inlyta). He had stable disease on follow-up. Moderate diarrhea, well controlled with antidiarrheal medication, and mild fatigue were reported. After cycle 6, he developed fatigue, mild shortness of breath, and mild cough without chest pain. Pulse oximetry was 93% at rest. He had no fever and no recent sick contact, and he was up to date on his influenza vaccine. The infectious work-up result was negative. A chest CT scan at approximately week 18 confirmed grade 3 pneumonitis, and pembrolizumab was held. Oral steroids were administered. Ultimately, pembrolizumab was discontinued, but the patient continued axitinib.
Fourteen months after initiation of systemic therapy, the patient reported increasing back pain, mild nausea, weight loss, and new onset of persistent rib pain. Imaging confirmed disease progression: growth of paratracheal lymph node (from 20 × 15 mm to 25 × 28 mm), new mediastinal and hilar nodal involvement, new retroperitoneal nodes, and new lytic osseous lesions. His ECOG performance status was 1.
Cabozantinib (Cabometyx) was initiated. Nine months later, disease progression was documented. The patient would like to continue active therapy.
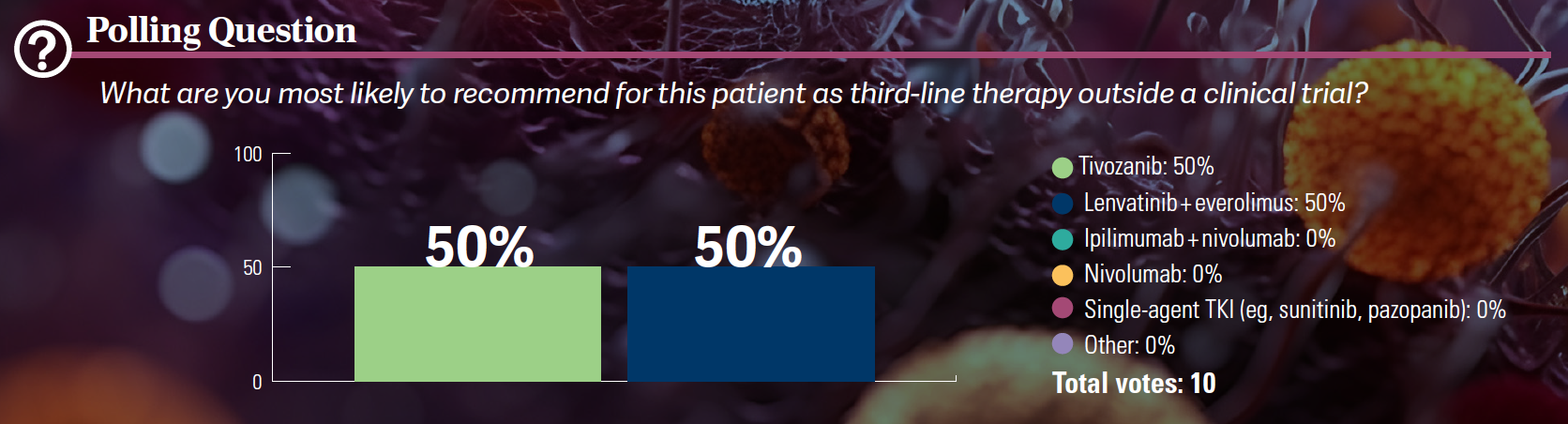
DISCUSSION QUESTIONS
- Have you used tivozanib (Fotivda)?
- What are your impressions of this agent?
BARATA: This is relatively recent, and [some of us] might not be aware of this therapy as an option. For those of you who have not used it, [what is] the reason why you have not used it that you want to share?
SALIB: The drug has been approved [since 2021],…but because we don’t have the right patient, maybe we need to wait for someone.
BARATA: So you didn’t have the opportunity to use it yet.
IYER: [For] my most recent patient, I was debating between lenvatinib [Lenvima] plus everolimus [Afinitor] and tivozanib. The decision to use lenvatinib/everolimus in that patient was because their performance status was quite good, and…when I looked at the adverse event [AE] profiles, it seemed like lenvatinib/everolimus would not be as well tolerated. I wanted to use that in an earlier-line setting before tivozanib and offer them that first and then later on if they progressed, maybe try tivozanib.
BARATA: So you’re thinking almost like toxicity drove your decision to…see [whether] this patient can [receive] both lenvatinib/everolimus and tivozanib, and [since] tivozanib seems be to tolerated better, save it for later.
DISCUSSION QUESTIONS
- What is your experience with lenvatinib plus everolimus?
- How do you approach dosing?
BARATA: Dr Iyer, you mentioned you would prioritize lenvatinib/everolimus [in a more fit patient] because of the toxicity and then [use] tivozanib later…. Is that the experience you have with lenvatinib/everolimus? And do you start them at 18-mg lenvatinib plus 5-mg everolimus, or do you use a different dose?
IYER: For my last patient, I started on 14-mg [lenvatinib] because I’ve used lenvatinib across the different tumor types, and [although] 18 mg is not the highest [dose]—like in uterine cancer [where] it’s even higher than that—I find lenvatinib to not be very well tolerated at higher doses.1 I started him on 14 mg. He did OK, but I only saw him for a few weeks before I moved to another office, so I couldn’t [ follow up with him] long term on that treatment.
BARATA: We hear that a lot about sometimes going [to a] lower dose. Does that concern you at all when we have a trial that shows the activity for 18 mg,2 and then we go into a lower dose? How much of that impacts your thought process that it might not work as well? Or [do you think], “It’s just too much, and I think with 14 mg, I might gain a lot of benefit from getting a lower dose”? How does that impact your thought process?
IYER: This might be extrapolating excessively, but I’m thinking about the ReDOS trial [NCT02368886] in colon cancer with regorafenib [Stivarga], where they started off at a lower dose and escalated up to the full treatment dose, and...tolerability was significantly better compared with starting at the full dose and efficacy was exactly the same.3 This is in colon cancer, which is a different tumor type, and it’s a different medication, which is regorafenib. Applying that similar principle, I’ve done that across tumor types—tried to start a bit lower in the hopes of getting them to the appropriate dose, rather than starting them at the full dose and having them crash after 3 weeks and then saying, “I’m never going to touch this drug again.” It doesn’t feel great, but at least there is something out there that gives me some justification to start lower.
MALHOTRA: I have used lenvatinib for [patients with other diseases], but I have not used this combination for RCC yet. The long-term PFS [progression-free survival] with tivozanib was something that caught my attention. I will probably favor tivozanib. As treatment lines go on, it is harder and harder for patients to [tolerate] treatment. If my patient still did not have too many gastrointestinal AEs from previous treatments, I would lean toward pursuing tivozanib.
BARATA: After 18-mg lenvatinib plus 5-mg everolimus got approved, it was mandated to run a noninferiority study that Sumanta K. Pal, MD, [of City of Hope] presented 2 years ago and then published last year, which compared lenvatinib 14 mg vs 18 mg [with] 5-mg everolimus, and…we have safety and efficacy data. If you look at the efficacy, the study was not noninferior, so it did not meet the primary end point [Table 14]. [For the 14-mg dose, there was a] 32% response rate at 6 months vs 35% for 18 mg, the standard dose. What was interesting was…numerically the rates of any grade 3 or higher AEs or intolerable grade 2 AEs within the 6-month period [were] actually numerically higher for the lower dose, 83% compared with 80% for 18-mg lenvatinib/5-mg everolimus, so the study was not [positive].4
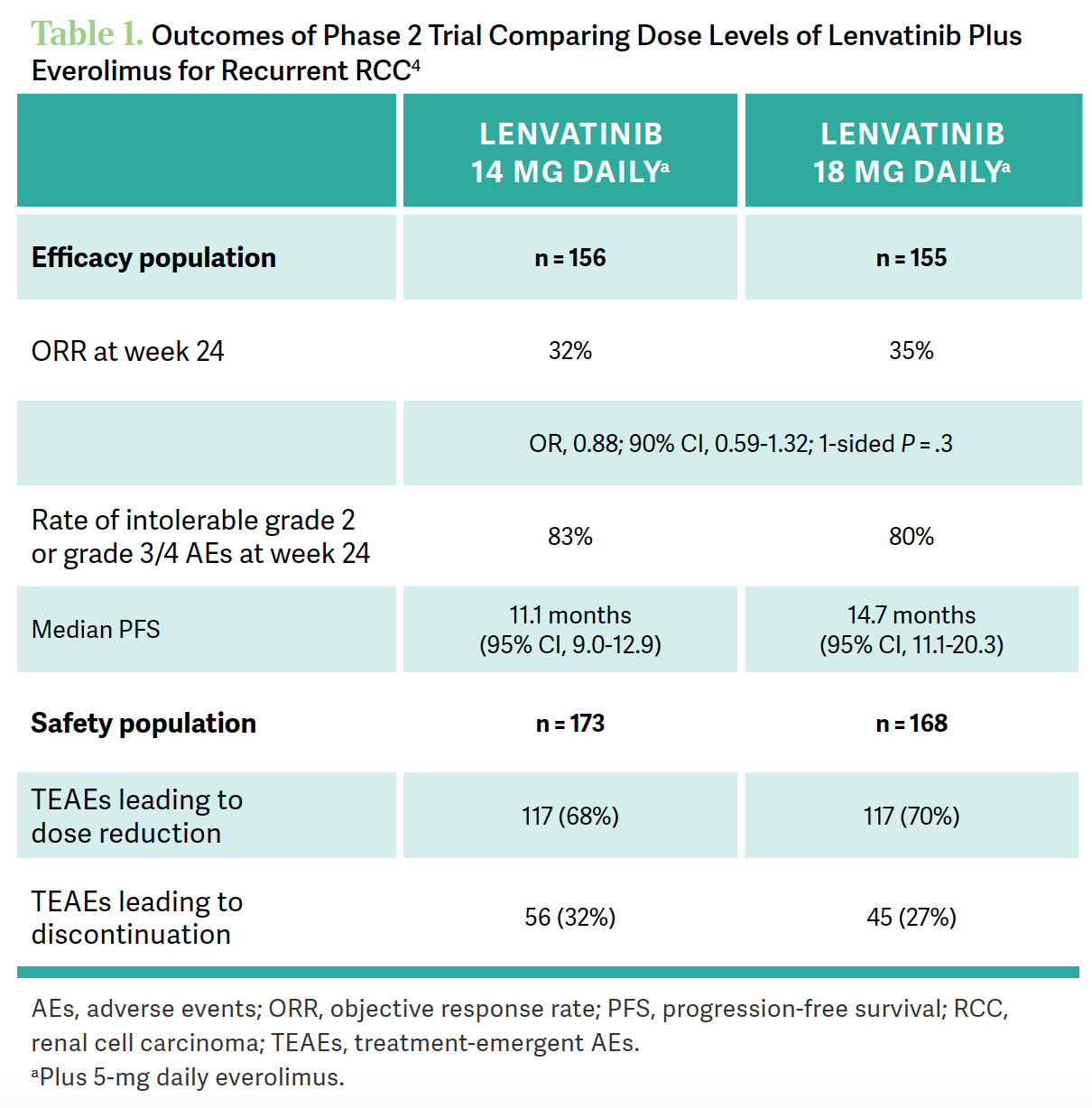
The study did not demonstrate that a lower dose was equivalent to the higher dose. The safety [results]… were, in general, similar. It appears that, at least in this trial, patients on the lower dose [of lenvatinib] did not benefit from a safety perspective when going on a lower dose compared with the higher dose. Also, if you look at the [median] PFS, numerically, it was 11.1 months for 14-mg lenvatinib compared [with] 14.7 months for the 18 mg. It appears that going down on the dose wasn’t necessarily a good idea in this trial.
APOSTOLOVA: How does one explain [there being] similar or more AEs with the lower dose?
BARATA: I don’t have a good explanation other than saying that for some patients, even 14 mg of lenvatinib is sometimes not well tolerated. That’s what was seen in that trial. I’ve heard that before. [Have you] had that same experience? Or have you tried 14 mg, and has it been successful?
APOSTOLOVA: No, I haven’t. I’ve given it once to a young patient [with] a lot of comorbidities and history of liver transplant, so I did a low dose.
DISCUSSION QUESTIONS
- What toxicities are most concerning to you in this setting? Which do you find most challenging to manage?
- How do you prevent, monitor, mitigate, and manage tyrosine kinase inhibitor (TKI)–associated toxicities?
BARATA: [We are looking at AEs] from their respective trials, so the goal is not to compare across studies. But I think it’s fair to say that if you look at the numbers, in general, [when] we look at the numbers regarding the fatigue, the hand-foot syndrome, and the diarrhea, the numbers on the more recent or the third-generation [TKIs] tivozanib and axitinib seem to be lower than cabozantinib and lenvatinib/everolimus. On [hypertension], they seem to be equivalent, but on the gastrointestinal AEs and the fatigue, they seem to be lower for tivozanib and for axitinib. We’re not comparing across trials; this is just the sense that we’re getting by looking at those numbers and how often patients are reporting those all-grade and significant AEs [Table 22,5-7].
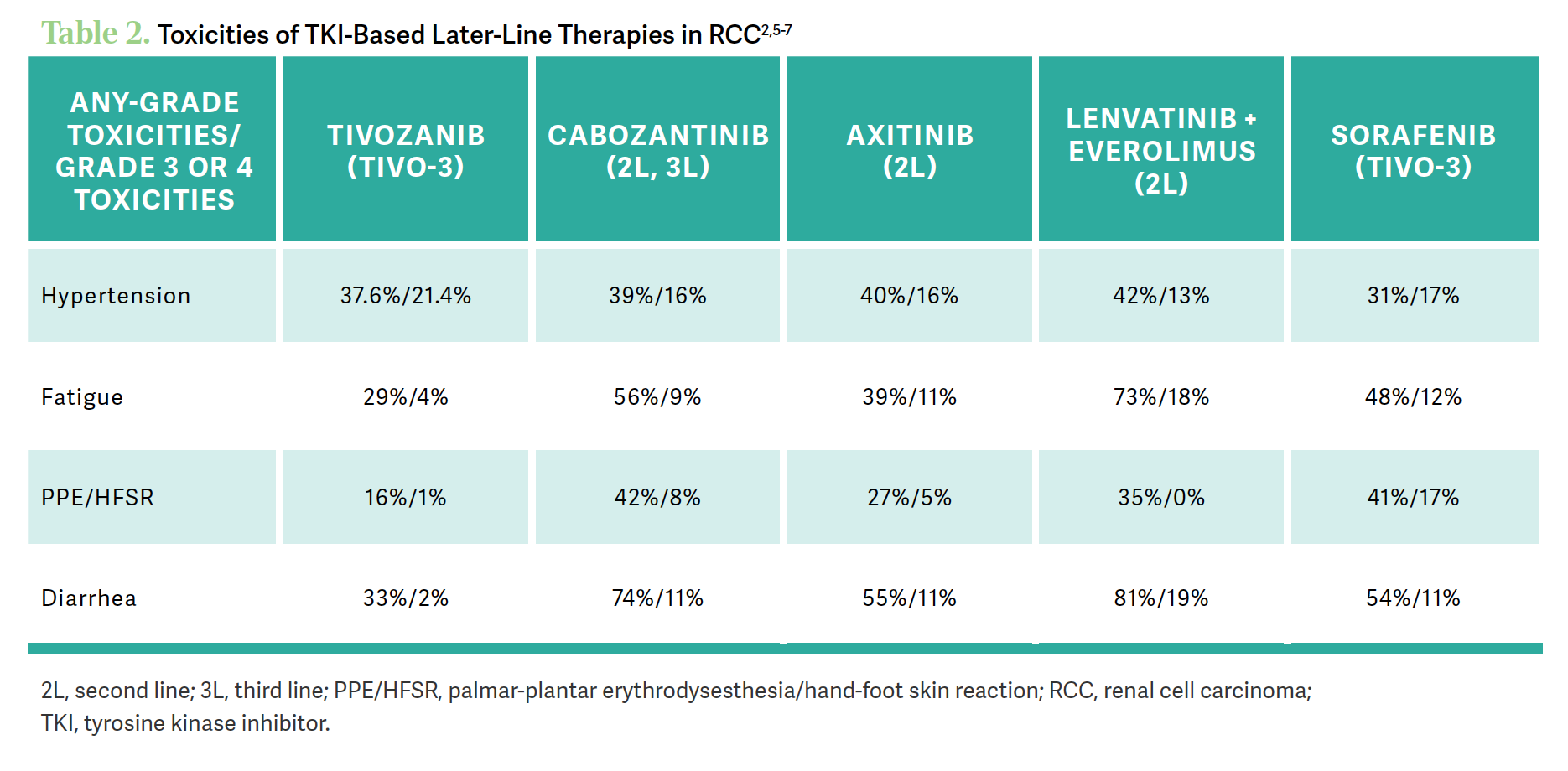
Dr Nathan, is that the sense that you get from the different options, and what do you do when your patients are developing these kinds of AEs?
NATHAN: I’ve used lenvatinib [and] cabozantinib. I was surprised at the lenvatinib/everolimus data—the fatigue is 73% and 81% diarrhea2—but I haven’t experienced it. But I always prepare my patients for any AEs. So if I anticipate diarrhea, I give them diphenoxylate/atropine [Lomotil] and loperamide [Imodium] and [avoid] mild, moderate, and severe diarrhea. I didn’t encounter a lot of these AEs. Hypertension is a concern, and I will make sure that I work with the primary care physician and the cardiologist, whoever is managing their blood pressure, to take care of their hypertension. We have work-arounds for these AEs; I am more focused on the efficacy.
BARATA: Got it. And you respond to these expected AEs by proactively working with the patient and making them aware of those, and act on them. That is very helpful.
REFERENCES
1. Lenvima. Prescribing information. Eisai Inc; 2022. Accessed October 24, 2023. https://tinyurl.com/bdz5z5fs
2. Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16(15):1473-1482. doi:10.1016/ S1470-2045(15)00290-9
3. Bekaii-Saab TS, Ou FS, Ahn DH, et al. Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20(8):1070-1082. doi:10.1016/S1470-2045(19)30272-4
4. Pal SK, Puente J, Heng DYC, et al. Assessing the safety and efficacy of two starting doses of lenvatinib plus everolimus in patients with renal cell carcinoma: a randomized phase 2 trial. Eur Urol. 2022;82(3):283-292. doi:10.1016/j.eururo.2021.12.024
5. Fotivda. Prescribing information. AVEO Pharmaceuticals Inc; 2021. Accessed October 24, 2023. https://tinyurl.com/bddpm79c
6. Cabometyx. Prescribing information. Exelixis Inc; 2021. Accessed October 24, 2023. https://tinyurl.com/526xwut2
7. Inlyta. Prescribing information. Pfizer Inc; 2022. Accessed October 24, 2023. https://tinyurl.com/5y2fzyvs

Enhancing Precision in Immunotherapy: CD8 PET-Avidity in RCC
March 1st 2024In this episode of Emerging Experts, Peter Zang, MD, highlights research on baseline CD8 lymph node avidity with 89-Zr-crefmirlimab for the treatment of patients with metastatic renal cell carcinoma and response to immunotherapy.
Listen
Beyond the First-Line: Economides on Advancing Therapies in RCC
February 1st 2024In our 4th episode of Emerging Experts, Minas P. Economides, MD, unveils the challenges and opportunities for renal cell carcinoma treatment, focusing on the lack of therapies available in the second-line setting.
Listen
Gasparetto Explains Rationale for Quadruplet Front Line in Transplant-Ineligible Myeloma
February 22nd 2025In a Community Case Forum in partnership with the North Carolina Oncology Association, Cristina Gasparetto, MD, discussed the CEPHEUS, IMROZ, and BENEFIT trials of treatment for transplant-ineligible newly diagnosed multiple myeloma.
Read More