Exploring First-line Bevacizumab in Ovarian Cancer
Thomas C. Krivak, MD, interpreted the results clinical trials of bevacizumab as treatment of patients with ovarian cancer to determine the optimal treatment strategy for a 69-year-old patient.
Thomas C. Krivak, MD

Thomas C. Krivak, MD, director, Ovarian Cancer Center of Excellence, and cochair, Society of Gynecologic Oncology Research Institute, Allegheny Health Network, interpreted the results clinical trials of bevacizumab as treatment of patients with ovarian cancer to determine the optimal treatment strategy for a 69-year-old patient.
Kirvak discussed the treatment options with a group of peers during a Targeted Oncology Case Based Peer Perspectives event.
Targeted Oncology: What type of testing would you order at this point?
KRIVAK: Some might consider ordering a panel test or a tumor test. You have both in your armamentarium due to the fact that you have tumor as well as germline samples. You can order a panel test, and if it is negative, you can send for an HRD test. If the results of the BRCA test are negative, we’ll send for an HRD test.
What are the therapeutic options for a patient with advanced ovarian cancer that is BRCA wild type and HRD?
If the patient were HRD, I would consider a PARP inhibitor. The decision about PARP inhibitor monotherapy or in combination with bevacizumab [Avastin] depends on the patient. But I think it boils down to the fact that we have lots of choices. I am biased toward bevacizumab, so I would have had this patient on bevacizumab and would have added olaparib [Lynparza] to that. I think it’s great that we have choices now.
The SOLO-1 trial [NCT01844986] only captured data [in] about 15% or 20% of our patients.1 Now we can treat these folks. To me, we’re making progress.

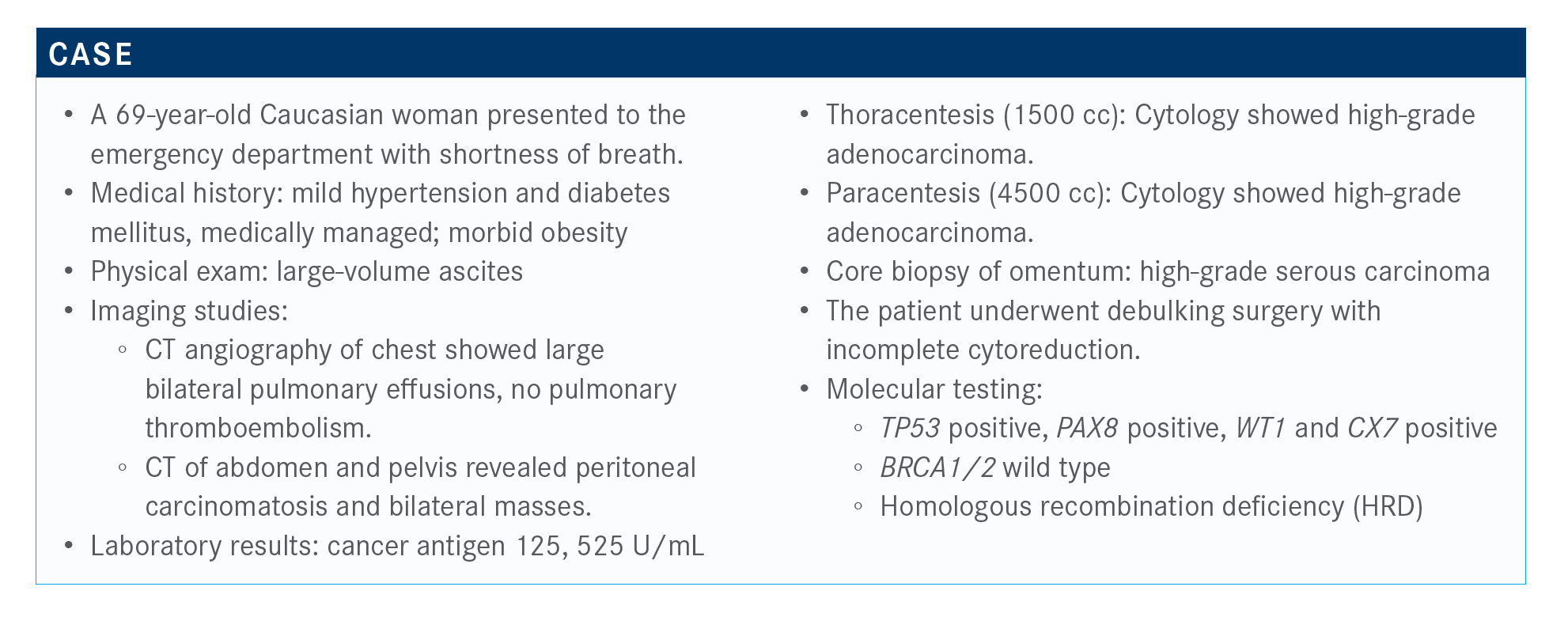
How do you interpret these results?
The majority of clinicians would treat with olaparib plus bevacizumab, single-agent olaparib, or single-agent niraparib [Zejula]. This is a great question. All those are correct answers. I look at this list, and I would probably rate, in my mind, choice 5 as No. 1, then choice 3, choice 4, choice 2, and then choice 1. That’s how I would rank them, but that’s just me. I think that’s my goal as a clinician, [and] I think we’ve now moved on to just about every patient should have or at least be offered and encouraged to participate in maintenance therapy.
What are the treatment options according to the National Comprehensive Cancer Network (NCCN) guidelines?
It all depends on the patient. That’s what the treatment choices depend on how they’re targeted, what medications they’re on, what some of the adverse effects are with the up-front therapy. The NCCN guidelines say that if you use bevacizumab as part of the up-front therapy, with BRCA1/2 wild type unknown, [and there is a] complete or partial response, you can go with bevacizumab plus olaparib or bevacizumab alone.2 The NCCN guidelines basically took the ITT [intention-to-treat] portion of PAOLA-1 [NCT02477644] and designated that all patients can receive this.3 If they’re germline or with somatic BRCA1 and BRCA2 mutations on bevacizumab, you can add olaparib, or change over to single-agent olaparib, or change over to single-agent niraparib. To me, these guidelines are consistent with [my practice].
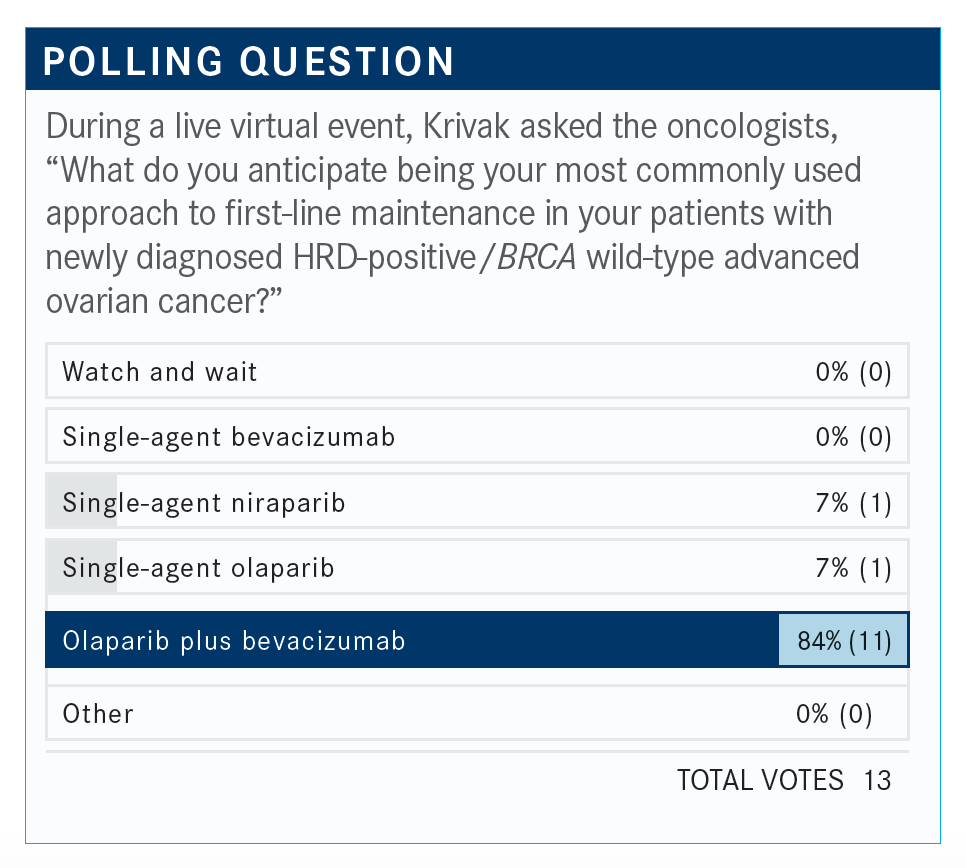
How do you interpret these findings?
This patient was 69 years of age. Nobody selected choice 4, so I take that as everybody would do some type of maintenance—50% chose bevacizumab, 33% chose bevacizumab plus olaparib, 16% chose niraparib.
All 4 answers are correct. Again, if the patient has a poor functional status, I would probably go with single-agent bevacizumab over single-agent niraparib at this point. This is the most complex issue that needs further investigation. I’m not convinced of the single-agent niraparib data from the PRIMA study [NCT02655016].4 I know it’s positive, and it was positive by a few months, but I think the homologous recombination proficient group is the one that we have to study the most.
How would you sequence these therapies?
Sequencing [is] another challenging question to think about. I don’t think we know how to sequence in recurrent ovarian cancer well. That’s definitely something that we need to get better at or get more consistent. When the patient is on bevacizumab, some oncologists might wonder if that is going to define platinum-sensitive or platinum-resistant disease.
References:
1. Moore K, Colombo N, Scambia G, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379(26):2495-2505. doi:10.1056/NEJMoa1810858
2. NCCN. Clinical Practice Guidelines in Oncology. Ovarian cancer including fallopian tube cancer and primary peritoneal cancer, version 1.2020. Accessed November 2, 2020. https://bit.ly/35ZX9PA
3. Ray-Coquard I, Selle F, Harter P, et al. PAOLA-1: an ENGOT/GCIG phase III trial of olaparib versus placebo combined with bevacizumab as maintenance treatment in patients with advanced ovarian cancer following fi rst-line platinum-based chemotherapy plus bevacizumab. J Clin Oncol. 2016;34(suppl 15):TPS5607. doi:10.1200/JCO.2016.34.15_suppl.TPS5607
4. González-Martín A, Pothuri B, Vergote I, et al; PRIMA/ENGOT-OV26/GOG-3012 Investigators. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381(25):2391-2402. doi:10.1056/NEJMoa1910962








