Effective Management of CINV Improves Response to Treatment
The goal of selecting optimal antiemetic therapy continues to be a moving target with the emergence of newer agents and patient-related risk factors, as well as the rapid evolution of guidelines for the management of chemotherapy-induced nausea and vomiting.
5-HT3 Receptor Antagonists
The first-generation 5-HT3 receptor antagonists (RAs; ondansetron, granisetron, dolasetron [Anzemet], and tropisetron [Navoban]) revolutionized the management of CINV in terms of efficacy and safety compared with historical antiemetics (eg, metoclopramide [Reglan]).1These agents exhibit comparable efficacy, preventing 50% to 80% of acute CINV in patients receiving moderately emetogenic chemotherapy (MEC) or highly emetogenic chemotherapy (HEC) regimens and are considered equivalent in CINV guidelines. Despite their efficacy against acute CINV, these agents are much less effective at preventing delayed CINV.
In contrast, the second-generation 5-HT3 RA, palonosetron (Alexi), demonstrates efficacy in preventing both acute and delayed CINV. Palonosetron plus dexamethasone has demonstrated superiority over the first-generation 5-HT3 RAs plus dexamethasone.1,2These advantages result from the unique pharmacodynamic properties of palonosetron, including a longer half-life and higher receptor binding affinity compared with the first-generation 5-HT3 RAs. In addition, binding of palonosetron to the 5-HT3 receptor creates positive cooperativity that results in further palonosetron binding and eventual receptor internalization, blocking 5-HT3 signaling and crosstalk with NK-1 receptor signaling.3,4
A novel extended-release subcutaneous formulation of granisetron, APF530, was recently approved by the FDA for CINV.5A unique biopolymer delivery system provides sustained release to improve the therapeutic concentration of granisetron over an extended period (>5 days).
A phase III study compared APF530 with ondansetron, both in combination with fosaprepitant (Emend for injection) and dexamethasone in patients receiving HEC.6Delayed-phase complete response (CR) was significantly more common in patients receiving APF530 compared with patients treated with ondansetron (65% vs 57%;P= .014). The most common treatment-related adverse events were constipation, fatigue, headache, and infusion-site reactions.
Overview of NK-1 RAs
Development of neurokinin-1 (NK-1) RAs has greatly improved our ability to control CINV. Aprepitant (Emend) was the first NK-1 RA to be approved for CINV based on demonstrated protection against delayed CINV in patients receiving HEC or MEC.7-9The addition of aprepitant to ondansetron/dexamethasone significantly increased the proportion of patients who did not experience vomiting, but did not have a significant protective effect against nausea. This aprepitant triplet regimen also showed superior prevention of CINVthat is, no vomiting and a CRin patients receiving MEC in a phase III randomized trial compared with ondansetron/dexamethasone alone.9
The water insolubility of aprepitant led to the development of the prodrug, fosaprepitant, which is readily converted to aprepitant following intravenous (IV) administration.10,11A phase III noninferiority trial of 2247 patients receiving cisplatin-based chemotherapy then compared a single dose of IV fosaprepitant to the standard 3-day regimen of oral aprepitant, both in combination with ondansetron/dexamethasone.12CR was improved in the fosaprepitant arm for the delayed (78.9% vs 68.5%;P<.001) and overall (77.1% vs 66.9%;P<.001) phases in comparison with the aprepitant arm, but CR was not improved in the acute phase (93.2% vs 91.0%;P= .184).
Netupitant and NEPA
Preclinical study results revealed synergistic activity for netupitant (Akynnzeo), a novel NK-1 RA, and palonosetron that suggested that this combination could be highly effective against CINV.13NEPA300 (netupitant with palonosetron) was subsequently developed to provide a convenient single-dose antiemetic therapy that combined both drugs.
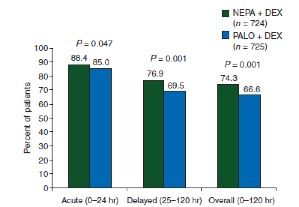
A randomized phase III trial directly compared a single dose of NEPA to a single dose of oral palonosetron (0.5 mg), both with dexamethasone, in 1455 patients receiving doxorubicin (Adriamycin) and cyclophosphamide (AC)based chemotherapy.14NEPA significantly increased the CR rate compared with palonosetron during the delayed phase (76.9% vs 69.5%;P= .001), acute phase (88.4% vs 85.0%;P= .047), and overall (74.3% vs 66.6%;P= .001) (Figure 123). NEPA provided more protection than palonosetron alone from both emesis and nausea in the delayed and overall phases. The oral fixed-dose NEPA capsule was approved by the FDA in 2014.
Rolapitant (Varubi)
Rolapitant, another novel NK-1 RA, was approved by the FDA in 2015 in combination with other antiemetic therapies for the prophylaxis of delayed CINV associated with initial and repeat courses of MEC or HEC. Two phase III trials evaluated rolapitant against granisetron in patients receiving HEC (HEC-1 and HEC-2), each of which enrolled over 500 patients.15In a pooled analysis of both studies, the addition of rolapitant to granisetron and dexamethasone significantly increased the percentage of patients who experienced a CR in the delayed phase (71% vs 60%;P= .0001).
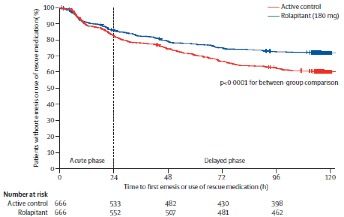
A third phase III study examined 1369 patients receiving MEC, randomizing them to rolapitant (180 mg orally on day 1) or placebo with granisetron (2 mg orally on days 1-3) and dexamethasone.16Of note, at the time of the study design, AC was considered an MEC and patients receiving this combination were included. Significantly more patients receiving rolapitant achieved a CR in the delayed phase (71% vs 62%;P= .0002) and the overall phase (69% vs 58%;P<.0001) compared with the control group (Figure 225).
Comparisons of NK-1 RAs
Differences in the pharmacokinetic properties of NK-1 RAs influence the dosage and use of these agents (Table).17-20Aprepitant and its prodrug fosaprepitant have a relatively short half-life of 9 to 13 hours, with aprepitant dosed daily for 3 days and fosaprepitant dosed only on day 1.17,18In contrast, NEPA and rolapitant have half-lives of approximately 80 and 180 hours, respectively, and both agents can be administered as a single dose prior to each chemotherapy cycle.19,20
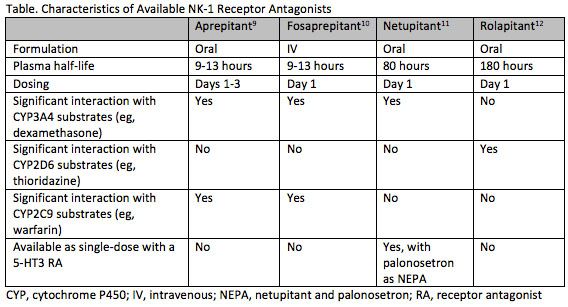
Another key difference between NK-1 RAs is their interaction with other drugs. Aprepitant, fosaprepitant, and netupitant induce or inhibit cytochrome P450 3A4 (CYP3A4), while rolapitant does not.17-20This interferes with the metabolism of CYP3A4 substrates, such as dexamethasone and some chemotherapeutic agents, requiring careful consideration and appropriate dose reductions of concomitant dexamethasone. Aprepitant and fosaprepitant also interact with warfarin and oral contraceptives. Rolapitant, on the other hand, inhibits CYP2D6, breast cancer resistance protein (BCRP), and p-glycoprotein.20
Olanzapine
Olanzapine is an atypical antipsychotic that blocks multiple neutrotransmitters and interacts with dopaminergic, serotonergic, adrenergic, and histamine receptors. A recent large randomized phase III trial evaluated the addition of olanzapine versus placebo to a standard triplet antiemetic regimen of aprepitant or fosaprepitant, a 5-HT3 receptor antagonist, and dexamethasone.21A total of 380 chemotherapy-naïve patients receiving HEC (cisplatin or cyclophosphamide/doxorubicin) were randomized to either olanzapine (10 mg orally) or placebo on days 1 through 4. The addition of olanzapine significantly improved the primary endpoint of no chemotherapy-induced nausea in the acute period (74% vs 45%;P= .002) and the delayed period (42% vs 25%;P= .002). Overall nausea prevention also significantly increased (37% vs 22%;P= .002). Side effects associated with olanzapine include short-term sedation, unwanted increased appetite, and weight gain. In this phase III trial, sedation was significantly increased in those receiving olanzapine, including severe sedation in 5% of patients.
Olanzapine has also been investigated as a rescue therapy for patients with breakthrough CINV. A double-blind randomized phase III trial directly compared olanzapine to metoclopramide in chemotherapy-naïve patients receiving HEC (cisplatin or doxorubicin/cyclophosphamide) who experienced breakthrough CINV despite prophylactic fosaprepitant, palonosetron, and dexamethasone.22Olanzapine prevented vomiting in 70% or patients compared with 31% with metoclopramide (P<.01). Nausea was also significantly reduced (68% vs 23%;P<.01).
Guideline Changes
Carboplatin, previously considered an MEC agent, was reclassified as a highly emetogenic agent when used at higher doses (AUC>4) by the National Comprehensive Cancer Network.23The American Society of Clinical Oncology24as well as the European Society of Medical Oncology and Multinational Association of Supportive care in Cancer25have also reclassified carboplatin at any dose as HEC. Guidelines now generally recommend a 4-drug regimen for HEC, including olanzapine, a 5-HT3 RA, an NK-1 RA, and dexamethasone.
New Questions
Although vomiting from chemotherapy has been largely abrogated by appropriate prophylaxis, nauseaparticularly delayed nausea—remains an unsolved issue. Is nausea triggered by other pathways that can be blocked? One potential class of agent that deserves more study is the cannabinoids, particularly with increased access now available. Will active agents in cannabis, such as tetrahydrocannabino, have a useful role in CINV? Why guidelines are not followed in a substantial minority of patients remains an elusive question and worthy of study. How can we incorporate patient risk factors into CINV prophylaxis choices, particularly in MEC? Future clinical trials will need to assess novel antiemetic therapies further, with the goal of complete control of all aspects of CINV. This will greatly improve quality of life for patients and will provide clinicians with the information they need to select optimal CINV prophylaxis from the start.
References:
- Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;374(4):1356-1367. doi: 10.1056/NEJMra1515442.
- Navari RM. Palonosetron for the prevention of chemotherapy-induced nausea and vomiting: approval and efficacy. Cancer Manag Res. 2009;1:167-176.
- Rojas C, Raje M, Tsukamoto T, Slusher BS. Molecular mechanisms of 5-HT(3) and NK(1) receptor antagonists in prevention of emesis. Eur J Pharmacol. 2014;722:26-37. doi: 10.1016/j.ejphar.2013.08.049.
- Rojas C, Stathis M, Thomas AG, et al. Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg. 2008;107(2):469-478. doi: 10.1213/ane.0b013e318172fa74.
- Granisetron [prescribing information]. Heron Therapeutics: Redwood City, CA; 2016. sustol.com/public/pdfs/PI.pdf. Accessed: June 26, 2017.
- Schnadig ID, Agajanian R, Dakhil C, et al. APF530 (granisetron injection extended-release) in a three-drug regimen for delayed CINV in highly emetogenic chemotherapy. Future Oncol. 2016;12(12):1469-1481. doi: 10.2217/fon-2016-0070.
- Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al; Aprepitant Protocol 054 Study Group. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003;97(12):3090-3098. doi: 10.1002/cncr.11433.
- Hesketh PJ, Grunberg SM, Gralla RJ, et al; Aprepitant Protocol 052 Study Group. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin--the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21(22):4112-4119. doi: 10.1200/JCO.2003.01.095.
- Rapoport BL, Jordan K, Boice JA, et al. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: a randomized, double-blind study. Support Care Cancer. 2010;18(4):423-431. doi: 10.1007/s00520-009-0690-9.
- Grunberg S, Chua D, Maru A, et al. Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with cisplatin therapy: Randomized, double-blind study protocolEASE. J Clin Oncol. 2011;29(11):1495-1501. doi: 10.1200/JCO.2010.31.7859.
- Lasseter KC, Gambale J, Jin B, et al. Tolerability of fosaprepitant and bioequivalency to aprepitant in healthy subjects. J Clin Pharmacol. 2007;47(7):834-840. doi: 10.1177/0091270007301800.
- Weinstein C, Jordan K, Green SA, et al. Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with moderately emetogenic chemotherapy: Results of a randomized, double-blind phase III trial. Ann Oncol. 2016;27(1):172-178. doi: 10.1093/annonc/mdv482.
- Stathis M, Pietra C, Rojas C, Slusher BS. Inhibition of substance P-mediated responses in NG108-15 cells by netupitant and palonosetron exhibit synergistic effects. Eur J Pharmacol. 2012;689(1-3):25-30. doi: 10.1016/j.ejphar.2012.05.037.
- Aapro M, Rugo H, Rossi G, et al. A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol. 2014;25(7):1328-1333. doi: 10.1093/annonc/mdu101.
- Rapoport BL, Chasen MR, Gridelli C, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: Two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncol. 2015;16(9):1079-1089. doi: 10.1016/S1470-2045(15)00035-2.
- Schwartzberg LS, Modiano MR, Rapoport BL, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of moderately emetogenic chemotherapy or anthracycline and cyclophosphamide regimens in patients with cancer: A randomised, active-controlled, double-blind, phase 3 trial. Lancet Oncol. 2015;16(9):1071-1078. doi: 10.1016/S1470-2045(15)00034-0.
- Aprepitant [prescribing information]; Merck Sharp & Dohme Corp: Whitehouse Station, NJ. 2017. Available at: merck.com/product/usa/pi_circulars/e/emend/emend_pi.pdf. Accessed May 30, 2017.
- Fosaprepitant dimeglumine [prescribing information]; Merck Sharp & Dohme Corp: Whitehouse Station, NJ. 2016. Available at: merck.com/product/usa/pi_circulars/e/emend_iv/emend_iv_pi.pdf. Accessed May 30, 2017.
- Netupitant and palonosetron [prescribing information; Helsinn Therapeutics: Iselin, NJ. 2016. Available at: www.akynzeo.com/assets/pdf/Prescribing_Information.pdf. Accessed May 30, 2017.
- Rolapitant [prescribing information]; Tesaro, Inc: Waltham, MA. 2015. Available at: varubirx.com/downloads/VARUBI_(rolapitant)_Full_Prescribing_Information.pdf. Accessed May 30, 2017.
- Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375(2):134-142. doi: 10.1056/NEJMoa1515725.
- Navari RM, Nagy CK, Gray SE. The use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Cancer. 2013;21(6):1655-1663. doi: 10.1007/s00520-012-1710-6.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Antiemesis. Version 2.2017. Available at: nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed May 30, 2017.
- Hesketh PJ, Kris MG, Basch E, et al; American Society of Clinical Oncology. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(28):3240-3261. doi: 10.1200/JCO.2017.74.4789.
- Roila F, Molassiotis A, Herrstedt J, et al; participants of the MASCC/ESMO Consensus Conference Copenhagen 2015. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(suppl 5):v119-v133. doi: 10.1093/annonc/mdw270.