Deep PSA Response With Apalutamide Linked to HRQOL Improvement in Advance Prostate Cancer
A look back at apalutamide treatment in the the TITAN and SPARTAN trials showed that deep prostate-specific antigen responses lead to improvement in certain health-related quality of life factors.

The deep PSA responses observed with the androgen receptor inhibitor with apalutamide (Erleada) correlated with beneficial outcomes in patient-related end points, according to findings from a post-hoc analysis of data from the phase 3 TITAN (NCT02489318) and SPARTAN (NCT01946204) trials presented in a poster at the 2022 Genitourinary Cancers Symposium in San Francisco, California.1
“Not only does a deep PSA response identify patients who will have improved survival and progression-free outcomes, but also it is also associated with maintenance of health-related quality of life, improved patient reported physical wellbeing, and a reduced risk of worsening pain and fatigue intensity,” said Eric Jay Small, MD, study investigator, coleader of the UCSF Prostate Cancer Program and deputy director of the UCSF Helen Diller Family Comprehensive Cancer Center.
Small said that previous results from TITAN and SPARTAN showed that apalutamide plus androgen depravation therapy (ADT) was associated with improved overall survival (OS), preserved health-related quality of life (HRQoL), and rapid and deep reductions in PSA. In this analysis, he and his colleagues set out to determine the relationship between PSA decline and HRQoL following apalutamide.
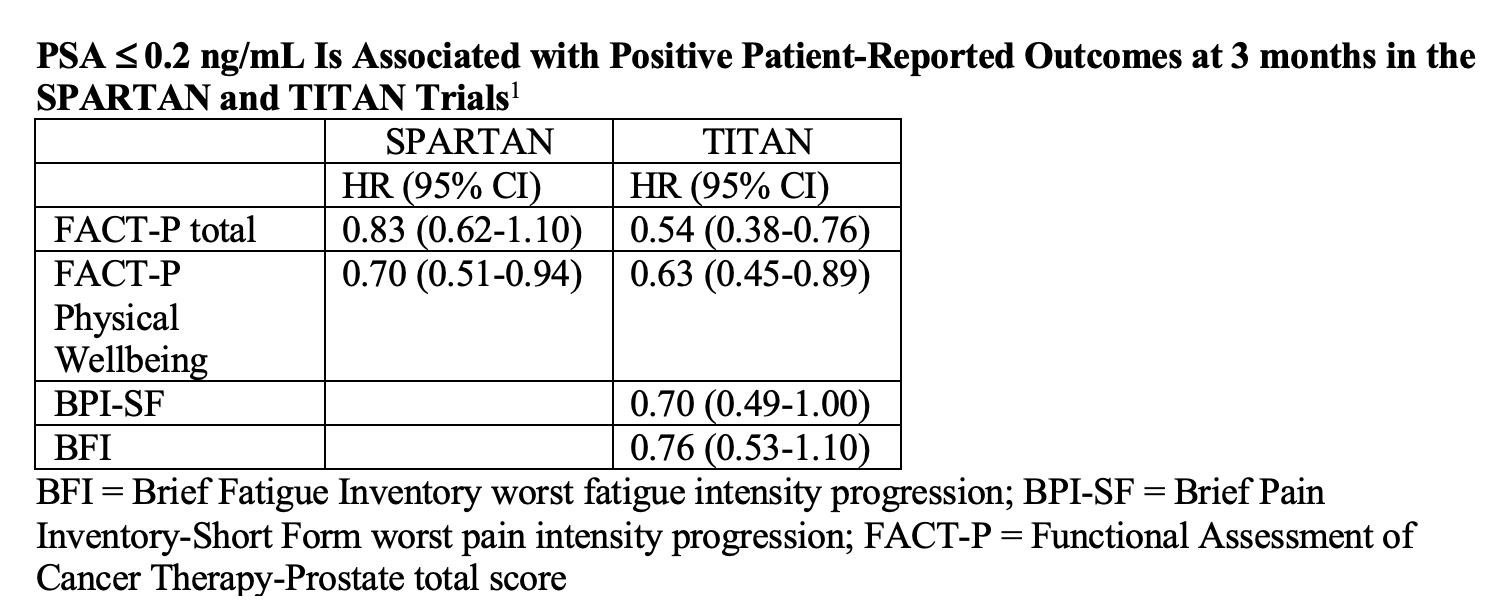
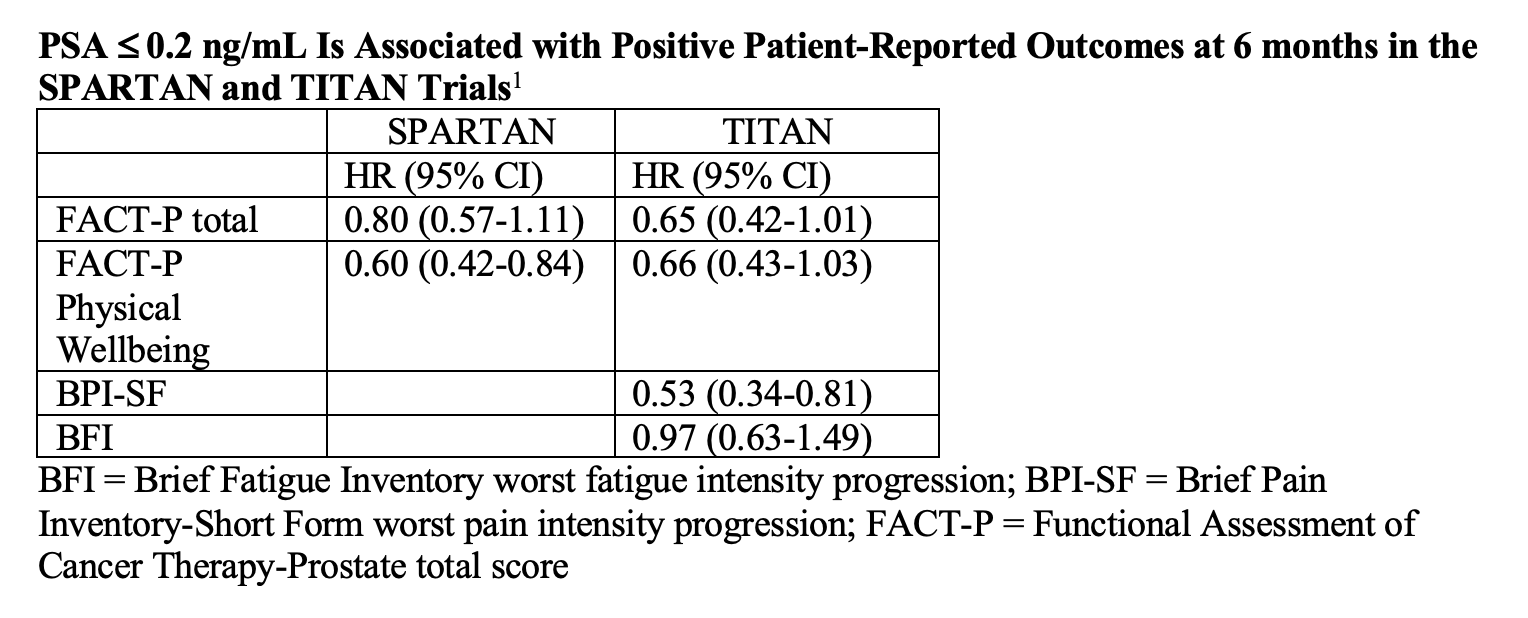
Investigators assessed HRQoL in the 2 trials separately because each had “distinct” patient populations with different disease burdens and different prior therapies. Small said that at 3 and 6 months, patients whose PSA was at or below 0.2 ng/mL had prolonged time to deterioration in their Functional Assessment of Cancer Therapy-Prostate total (FACT-P) and FACT-P Physical Wellbeing scores on both studies. In TITAN, patients had better scores on the Brief Pain Inventory-Short Form (BPI-SF) worst pain intensity progression and Brief Fatigue Inventory (BFI) worst fatigue intensity progression (TABLE).
A total of 1052 patients with metastatic castration-sensitive prostate cancer enrolled in TITAN and were randomly assigned to apalutamide 240 mg daily plus or placebo. Both groups received standard ADT.2
In findings published in 2021, the median OS for patients treated with apalutamide was not reached (NR) compared with 52.2 months for patients assigned to apalutamide (HR, 0.65; 95% CI, 0.53-0.79; P < .0001). At 48 months, the OS rate was 65.1% vs 51.8% in favor of apalutamide.
Patients with nonmetastatic castration-resistant prostate cancer in SPARTAN were assigned to apalutamide (n = 806) or placebo (n = 401). At a median follow-up of 52 months, the median OS was 73.9 months (95% CI, 61.2-NR) in the apalutamide group compared with 59.9 months (95% CI, 52.8-NR) with placebo (HR, 0.78; 95% CI, 0.64-0.96; P = .016).3
The FDA approved apalutamide for this indication in February 2018 based on results from SPARTAN.4
Investigators presented real-world evidence from the SPARTAN trial at the 2021 American Urological Association Annual Meeting. The overall PSA response was 83.5% at 6 months after initiating apalutamide treatment and 86% at 12 months.5
References
1. Small EJ, Chi KN, Chowdhury S, et al. Association between patient-reported outcomes (PROs) and changes in prostate-specific antigen (PSA) in patients (pts) with advanced prostate cancer treated with apalutamide (APA) in the SPARTAN and TITAN studies. J Clin Oncol. 2022;40(suppl 6; abstr 73). doi: 10.1200/JCO.2022.40.6_suppl.073
2. Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021;39(20):2294-2303. doi:10.1200/JCO.20.03488
3. Smith MR, Saad F, Chowdhury S, et al. Apalutamide and overall survival in prostate cancer. Eur Urol. 2021;79(1):150-158. doi:10.1016/j.eururo.2020.08.011
7. FDA approves apalutamide for non-metastatic castration-resistant prostate cancer. News release. Updated May 3, 2018. Accessed February 17, 2022. https://bit.ly/33sXJrU
8. Lowentritt B, Brown G, Kernen K, et al. Real-world effectiveness and treatment adherence of apalutamide in non-metastatic castration-resistance prostate cancer patients. J Urol. 2021;206(suppl 1):e58. doi:10.1097/JU.0000000000001969.08