CheckMate 069 Trial Shows Robust Updated Results
At the ASCO 2015 Annual Meeting, F. Stephen Hodi, MD presented an analysis of the phase II CheckMate 069 trial covering objective response rate, progression-free survival, and safety in predefined subgroups, including those with poor prognostic factors.
F. Stephen Hodi, MD

F. Stephen Hodi, MD
The results of the phase II CheckMate 069 trial were published in theNew England Journal of Medicineearlier this year.1In a press release, F. Stephen Hodi, MD, director of the Melanoma Treatment Center and director of the Center for Immuno-Oncology at the Dana-Farber Cancer Institute in Boston, described the results as “unprecedented.”2At the ASCO 2015 Annual Meeting, Hodi presented an analysis of the trial covering objective response rate (ORR), progression-free survival (PFS), and safety in predefined subgroups, including those with poor prognostic factors.3
Reviewing the rationale for this combination trial, Hodi pointed out the nonoverlapping mechanisms of action of anti-cytotoxic T-lymphocyte antigen-4 (CTLA-4) inhibitors and programmed cell death 1 (PD-1) inhibitors, namely ipilimumab (IPI) and nivolumab (NIVO), and their independent successes in trials, exhibiting superiority to other agents.4,5Furthermore, Hodi cited the very successful phase I study of the combination of IPI and NIVO that achieved an ORR of 40% and an up to 80 % reduction in tumor volume at the maximum tolerated dose.6
CheckMate 069 Trial: Structure, Endpoints, Patient Demographics
In the double-blind CheckMate 069 trial patients were randomized 2:1 to receive 3 mg/kg of IPI and1 mg/kg of NIVO (n = 95) or the same dose of IPI with placebo (n = 47) once every 3 weeks for 4 doses. IPI was then discontinued and patients received NIVO or placebo at the same dose every 2 weeks, until disease progression or unacceptable toxicity. Inclusion criteria ensured that eligible patients were treatment naïve, had unresectable stage III or IV melanoma, and a tissue sample for programmed cell death ligand 1 (PD-L1) assessment. The study aimed to recruit 100 patients withBRAFwild-type (WT) and 50 patients withBRAFmutant (MT) tumors. Patients were excluded if they had active brain metastases, uveal melanoma, or serious autoimmune disease.1,3
The primary endpoint was ORR in patients withBRAF V600WT tumors. Secondary endpoints included PFS in patients withBRAFWT tumors, ORR, and PFS in patients withBRAFV600-MTpositive tumors, and safety.1,3
The study enrolled 142 patients, approximately half of whom were older than 65 years, and more than 60% in each arm were males. Approximately 80% of patients in each arm had stage IV disease, and about half the patients in each arm had M1c disease. Lactate dehydrogenase (LDH) levels were normal in about 75% of patients per treatment group. Expression levels of PD-L1 ≥5% andBRAF V600-MT were found in about 25% of patients in each arm.1,3
Efficacy
The primary endpoint, ORR, was 59% (95% confidence interval [CI], 48-69) for patients receiving NIVO and IPI (n = 95) versus 11% (95% CI, 4-23) for patients receiving IPI alone (n = 47). This was a highly significant difference (P<.0001).3
Percentages of patients achieving complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) are shown in Figure 1. Response assessments were not possible for 13 patients in each arm.3
Figure 1. Percentages of patients achieving complete response, partial response, stable disease, and progressive disease.
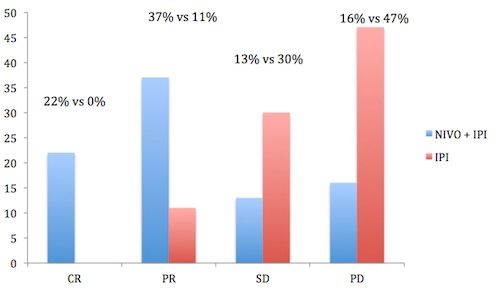
Figure 1. Percentages of patients achieving complete response, partial response, stable disease, and progressive disease.
Among responders, with a minimum follow-up of 11 months, there was an ongoing response in 46 of 56 (82%) patients in the NIVO and IPI group versus 4 of 5 (80%) patients in the IPI-alone group. Hodi drew attention to the finding that 30 of 44 (68%) patients who withdrew from the combination group because of drug-related toxicity achieved a CR or PR.1,3
Analysis of ORR for the specific subgroups of M1c disease, age <65 years/≥65 years, PD-L1 status ≥5%/<5%,BRAFstatus MT/WT, showed a statistically significant difference in favor of treatment with the NIVO + IPI combination versus IPI alone.3
Tumor burden showed a median reduction of 65.3 % in patients assigned to NIVO and IPI, whereas it increased by a median of 7.8% in patients assigned to IPI alone.1,3
Death or disease progression occurred in 42 of 95 patients in the NIVO + IPI arm versus 32 of 47 patients in the IPI-alone arm. Median PFS (among all randomized patients) was not reached for patients in the NIVO + IPI arm but was 3.0 (2.8-5.1) months in the IPI arm, with an HR of 0.39 (0.25-0.63, 95% CI,P<.0001).3
Safety
Of all patients receiving NIVO + IPI, 91% experienced treatment-related adverse events (AEs) of any grade, similar to the 93% reported by patients receiving IPI alone. However, 54% of patients on NIVO + IPI reported grade 3-4 AEs versus 24% of patients receiving IPI alone.1,3
The study also analyzed the incidence of treatment-related AEs in patient subgroups. The incidence of any grade treatment-related AEs in patients receiving NIVO + IPI who were <65 years, ≥65 years, or had M1c disease, were 90%, 94%, and 89%, respectively. The corresponding incidences for grade 3-4 AEs were 54%, 52%, and 59%. In patients receiving IPI alone, and analyzing the same subgroups, the incidences of any treatment-related AEs were similar to the NIVO + IPI arm, but the incidences of grade 3 to 4 AEs were lower at 26%, 15%, and 20% for each subgroup, respectively.3
The percentages of patients who discontinued therapy because of treatment-related AEs are shown in Figure 2.3There were 3 treatment-related deaths in the NIVO + IPI arm and none in the IPI-alone arm.3
The most common treatment-relatedselectAEs (%) were skin, gastrointestinal, and hepatic, followed by endocrine, and pulmonary, shown in Figure 3. Select AEs were defined as those with potential immune etiology. In addition, renal AEs of any grade were reported by 3% of patients receiving NIVO + IPI, 1% being grade 3 through 4. For patients receiving IPI alone, the incidences were reported as 2% and 0%.3
Figure 2. Percentages of patients who discontinued therapy because of treatment-related adverse events.
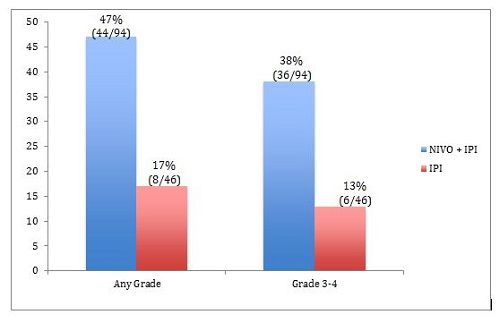
Figure 2. Percentages of patients who discontinued therapy because of treatment-related adverse events.
Figure 3. Most common treatment-related select AEs.
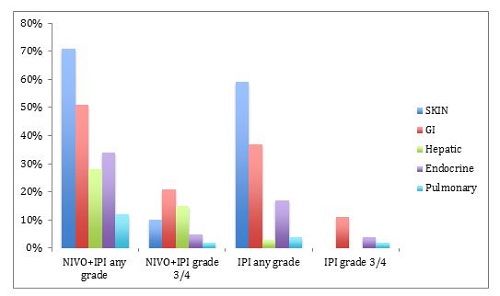
Figure 3. Most common treatment-related select AEs.
Reporting on the timing of the onset of the grade 3-4 AEs, Hodi pointed out that skin AEs peaked early at 2 to 3 weeks followed by gastrointestinal at 5 to 8 weeks. Endocrine AEs peaked at around 10 to 12 weeks, hepatic at 12 to 14 weeks, and pulmonary at 15 weeks. The renal AE occurred at 28 weeks. Most of the grade 3-4 AEs occurred during the combination phase of treatment.3
Most, about 80%, of the grade 3-4 treatment-related select AEs resolved at a median of 5 weeks after treatment with immune-modulating medications, except for the endocrinopathies. “Endocrinopathies frequently don’t resolve in patients given immune checkpoint blockade, as reported in multiple other studies,3” Hodi explained.
Conclusion
Summarizing the main points of the study, Hodi emphasized the superior efficacy outcomes for patients receiving NIVO + IPI versus IPI alone, and that the benefits in ORR and PFS were independent ofBRAFstatus, PD-L1 status, and the presence of poor prognostic factors. He stated that the NIVO + IPI combination has a favorable benefit/risk profile in patients with treatment-naïve advanced melanoma and that most treatment-related AEs were manageable following established guidelines and the use of immune modulators.3
The clinical trial program for this combination continues. Results from the ongoing phase III, double-blind CheckMate 067 (NCT01844505) study have recently been published.7It recruited previously untreated patients with unresectable or metastatic melanoma, randomized to NIVO monotherapy or NIVO + IPI versus IPI monotherapy.
Median PFS with the combination of NIVO + IPI was 11.5 months versus 2.9 months with IPI alone (HR, 0.42; 95% CI, 0.31-0.57;P<.001), at a follow up of ≥9 months. Single-agent NIVO also significantly delayed disease progression versus IPI monotherapy (HR, 0.57; 95% CI, 0.43-0.76;P<.001), achieving median PFS of 6.9 months.7
Commenting on the combined safety/efficacy aspects, of the NIVO + IPI approach, lead author Jedd D. Wolchok, MD, PhD, chief of the Melanoma and Immunotherapeutics Service at Memorial Sloan Kettering Cancer Center in New York City, said in conversation withTargeted Oncology, “Now, we can’t forget that 55% of patients receiving this combination had a grade 3 or 4 AE, and that has been consistent throughout the trials. However, the fact that we were able to deliver this therapy in 127 sites globally to 945 patients, one-third of whom received the combination with no treatment-related deaths, was very important. It showed that we could strike a good safety and efficacy balance.8”
References
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma [published online ahead of print April 20, 2015].N Engl J Med. doi:10.1056/NEJMoa141428.
- http://www.onclive.com/conference-coverage/aacr-2015/Adding-Nivolumab-to-Frontline-Ipilimumab-Improves-PFS-by-60-in-Melanoma. Accessed June 20, 2015.
- http://meetinglibrary.asco.org/content/144615-156. Accessed June 20, 2015.
- Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma.N Engl J Med. 2010;363:711-723.
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation.N Engl J Med. 2015;372:320-330.
- Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus ipilimumab in advanced melanoma.N Engl J Med. 2013;369:122-133.
- Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma [published online ahead of print May 31, 2015].N Engl J Med. PMID: 26027431.
- http://www.targetedonc.com/articles/Wolchok-Elaborates-on-NivolumabIpilimumab-Data-From-CheckMate-067. Accessed June 23, 2015.