Burris Calls for Greater Adoption of NGS by Community Oncologists
The development and advancements in targeted therapies and immunotherapies have dramatically changed drug development and clinical practice. With access to widespread genomic research and next-generation sequencing, details about somatic and germline mutations in solid tumors can better inform the treatment plan, Howard “Skip” Burris III, MD, explains.
Howard (Skip) Burris, III, MD

Howard (Skip) Burris, III, MD
The development and advancements in targeted therapies and immunotherapies have dramatically changed drug development and clinical practice. With access to widespread genomic research and next-generation sequencing (NGS), details about somatic and germline mutations in solid tumors can better inform the treatment plan. These details allow for clearer classification of tumor subtypes and subsequently lead to new drugs and targeted therapies.
“I’m a little bit surprised that we’re still debating the merits of NGS in 2019,” said Howard “Skip” Burris III, MD, chief medical officer, Sarah Cannon Research Institute, during the 2019 Community Oncology Alliance (COA) Annual Conference held in Orlando, Florida.1His surprise comes at a time when cancer treatment is evolving from one-size-fits-all to personalized medicine driven by biology. Yet, despite these advancements, the wider adoption of NGS in the clinic remains a puzzling challenge. Burris said that several factors could be contributing to the slow uptake: cost, quality of tissue/biopsy, turnaround time for results, rarity of applicability, and handling/interpretation of the data.
The amount of information that can be generated as a result of a patient whose tumor undergoes NGS is staggering and may be challenging to clinicians. As an example, Burris described the case of a 40-year-old patient with breast cancer who was estrogen receptor (ER)-positive, HER2-negative, and diagnosed in 1999. An NGS report was requested in 2019 and revealed her to be microsatellite stable. Her NGS results demonstrated the presence ofBRCA2,CHEK2,CDK4 amplification (20 copies),ESR1,PIK3CAE545K,PTENloss, andRB1loss. But this is such a huge amount of information that it can be daunting to understand, making education and incorporation overwhelming, Burris said.
In general, there are 3 types of molecular tests available, said Burris. Single-marker molecular tests such as immunohistochemistry, polymerase chain reaction, and fluores- cence in situ hybridization have been used for decades and will continue to play an important role in cancer diagnosis.
Multi-gene hot spot tests use NGS panels to identify pre-specified mutations occurring in very limited areas of genes of interest. This type of test could fail to detect all classes of genomic alterations, cautioned Burris.
Comprehensive genomic profiling tests all of the known clinically relevant cancer genes for all classes of alterations. “This is the only test to obtain tumor mutational burden [TMB] right now. The blood-based testing panels are coming along, and they’re going to have TMB in the near future, and some already have those reports out. But if you really want to get the whole assessment, this is the sort of test you need to order,” Burris said.
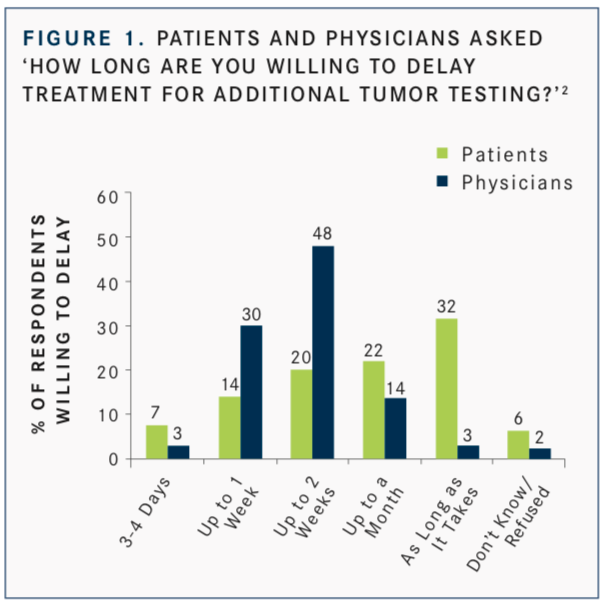
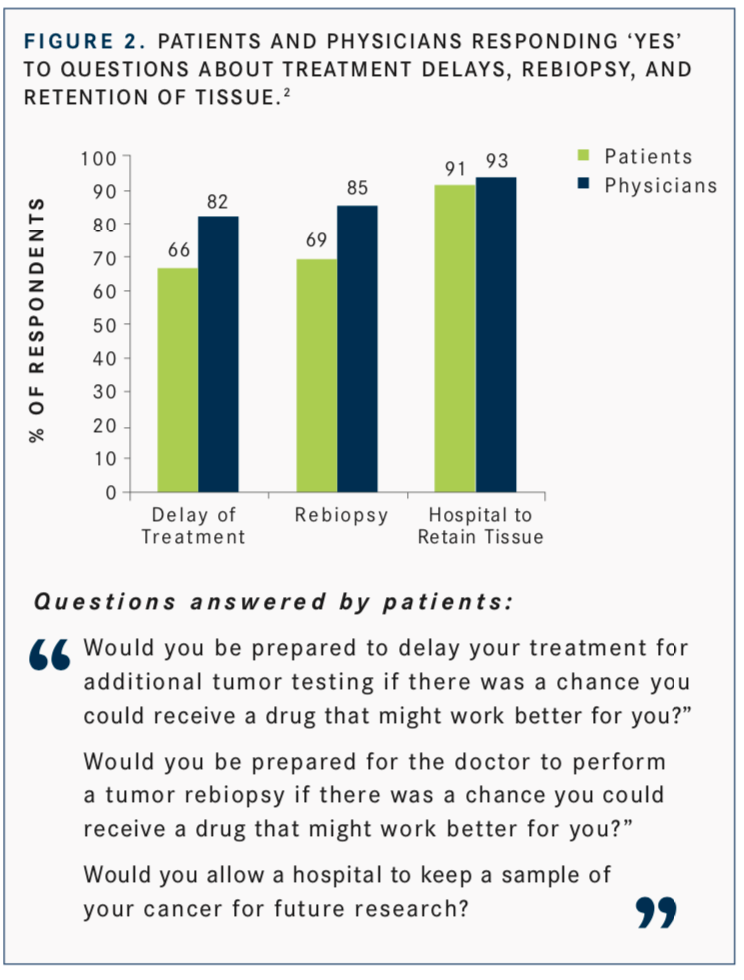
Interest in the use of NGS is not just clinician-focused, Burris said. In a study by Ciardiello F, Adams R, Tabernero J, et al,2patients responded to this question: “Would you be prepared to delay your treatment for additional tumor testing if there was a chance you could receive a drug that might work better for you?” Fifty-four percent of patients surveyed said they would be willing to delay treatment for up to a month or more to allow for additional tumor testing (FIGURE 1). This was over- estimated by physicians, 82% of whom believed that their patients would be willing to wait (FIGURE 2).
Burris is optimistic about the opportunity NGS represents for clinicians. The addition of new data and technologies will require clinicians to interpret and act on increasingly complex information. In addition, a number of standard- of-care treatments require knowledge of the existence and type of molecular alteration. The challenge currently is that molecular reports do not present information in an easily clinically actionable format.
“An NGS test should not be viewed in a similar fashion as buying a lottery ticket. In the lottery, you are purchasing 1 chance to win 1 combination of numbers. In NGS testing, you’re looking to assess a variety of genes, not just 1,” Burris said.TT
References
- Burris H. NGS: An overview for the practicing clinician in 2019. 2019 COA Annual Conference, April 3-5, 2019. Orlando, FL https://bit.ly/2HTvzsl.
- Ciardiello F, Adams R, Tabernero J, et al. Awareness, understanding, and adoption of precision medicine to deliver personalized treatment for patients with cancer: a multinational survey comparison of physicians and patients.Oncologist. 2016;21(3):292- 300. doi: 10.1634/theoncologist.2015-0279.

Conservative Management Is on the Rise in Intermediate-Risk Prostate Cancer
January 17th 2025In an interview with Peers & Perspectives in Oncology, Michael S. Leapman, MD, MHS, discusses the significance of a 10-year rise in active surveillance and watchful waiting in patients with intermediate-risk prostate cancer.
Read More
What Is Dark Zone Lymphoma, and Is It Clinically Relevant?
January 16th 2025Dark zone lymphoma includes aggressive B-cell lymphomas with shared molecular features. While some respond to escalated treatment, others remain resistant, highlighting the need for targeted approaches to improve outcomes.
Read More