BCMA-Targeting Agents for the Treatment of Multiple Myeloma Take Hold
Across therapy vehicles, agents targeting the B-cell maturation antigen have emerged as promising potential therapies in the multiple myeloma treatment paradigm. Following the 2019 American Society of Hematology Annual Meeting & Exposition, experts reflect on what these new advances mean for the field of oncology care.
Parameswaran Hari, MD, MRCP

Across therapy vehicles, agents targeting the B-cell maturation antigen have emerged as promising potential therapies in the multiple myeloma treatment paradigm. Following the 2019 American Society of Hematology Annual Meeting & Exposition, experts reflect on what these new advances mean for the field of oncology care.
“In the class of patients who are now known as triple-class refractory―refractory to an immuno-modulatory drug [IMiD], proteasome inhibitor [PI], and antibody―or ‘penta-refractory’―refractory to 2 IMiDs, 2 PIs, and daratumumab―we have essentially no good options except clinical trials,” Parameswaran Hari, MD, MRCP, professor and chief of the Division of Hematology Oncology at the Medical College of Wisconsin in Milwaukee, said in an interview with Targeted Therapies in Oncology.
Approximately 32,110 new cases of multiple myeloma were diagnosed in the United States in 2019, with about 50% of patients surviving at 5 years.1 Multiple myeloma is currently treated with mainly noncurative symptom management; thus, novel approaches are an area of active investigation.
Belantamab Mafodotin
Sagar Lonial, MD
During ASH, investigators for the randomized DREAMM-3 study described the phase III trial, which will compare belantamab mafodotin (GSK2857916) with pomalidomide (Pomalyst) and dexamethasone. The trial plans to enroll 320 participants, with a key primary end point of progression-free survival (PFS) and secondary end points including overall survival (OS), overall response rate (ORR), time toresponse, and the minimal residual disease (MRD)-negativity rate.2
“The results from the pivotal DREAMM-2 clinical study served as the basis for the [submission of the biologics license application (BLA)] to the FDA for the treatment of patients with relapsed/refractory multiple myeloma who were refractory to an immunomodulatory drug and a proteasome inhibitor and were refractory and/or intolerant to an anti-CD38 antibody. Treatment of patients with relapsed or refractory multiple myeloma remains challenging despite numerous therapeutic advances. Patients with disease refractory to immunomodulatory drugs, proteasome inhibitors, and anti CD38 monoclonal antibodies have a poor prognosis, with [available] agents resulting in 26% of patients achieving an overall response, a median progres-sionfree survival of 3.7 months, and a median overall survival of 8.6 months,” Ira Gupta, MD, MBBS, vice pres-ident and medicine development leader–belantamab mafodotin at GlaxoSmithKline, said in an interview.
In the DREAMM-2 study, whose results were recently published in Lancet Oncology, investigators observed responses in 31% of patients treated with belantamab mafodotin 2.5 mg/kg and in 34% of patients treated at the 3.4-mg/kg dose.3 The positive study results led to the BLA submission in December 2019.4
“Going forward, the value of belantamab mafodotin will likely be in combination with other existing therapeutic backbones, and earlier in the disease course, to exploit the potential for synergism, particularly as belantamab mafodotin is well tolerated and with a safety profile that is manageable and that does not overlap with [those] of other existing major therapeutic groups of drugs for multiple myeloma. However, this remains to be proven in clinical studies; a number of clinical studies combining belantamab mafodotin with other agents/backbone regimens are ongoing,” Hang Quach, MD, associate professor and director of Haematology Clinical Trials at St Vincent’s Hospital in East Melbourne, Australia, said in an interview.
In terms of clinical practice, Sagar Lonial, MD, chief medical officer of the Winship Cancer Institute of Emory University and professor and chair of the Department of Hematology and Medical Oncology at Emory University School of Medicine in Atlanta, Georgia, said the changes “will have an impact once the drug is approved. Similar to daratumumab [Darzalex], which was initially only approved in refractory multiple myeloma, there are currently trials ongoing that combine belamaf [belantamab mafodotin] with other active multiple myeloma agents such as lenalidomide [Revlimid], bortezomib [Velcade], and pomalidomide to bring this treatment to earlier lines of therapy to maximize the potential benefit for patients.”
CAR T-Cell Therapies
CARTITUDE-1
Deepu Madduri, MD

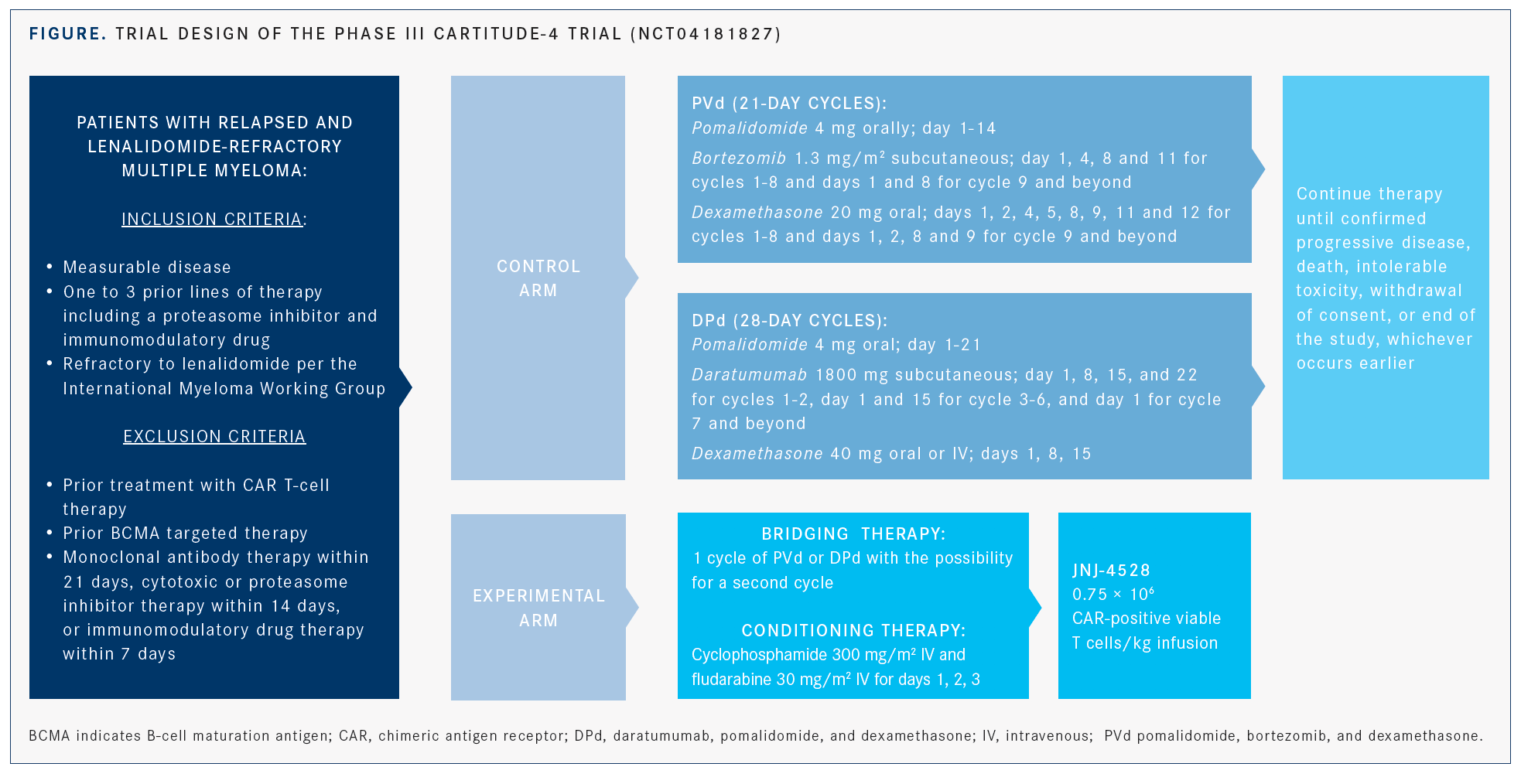
CARTITUDE-1 is an ongoing phase Ib/II trial examining the safety and efficacy of JNJ-68284528 (JNJ-4528), which leads to preferential expansion of CD8-positive (+) memory cells that target myeloma cells, in patients with relapsed/refractory multiple myeloma. The phase Ib results of this study were presented during the ASH meeting.5 JNJ-4528 is a structurally differentiated chimeric antigen receptor (CAR) with 2 single-domain antibod-ies directed at BCMA as well as a 4-1BB costimulatory domain.6 In the trial, the median administered dose was 0.73 × 106 CAR+ cells/kg (range, 0.5-0.9 × 106).
"JNJ-4528 was used in relapsed/refractory patients who had failed a median of 5 (and up to 18) prior lines of chemotherapy. It showed a 100% response rate with 69% complete remission or better rates," according to lead study author Deepu Madduri, MD, assistant professor of medicine (hematology and medical oncology) at The Mount Sinai Hospital in New York, New York.
CARTITUDE-1 enrolled 29 patients. All the patients evaluable for MRD response were MRD negative, and 27 patients remained progression free at the median follow-up of 6 months. Cytokine release syndrome (CRS) occurred in 27 (93%) patients but was mostly grade 1 or 2. Only 1 dose-limiting toxicity of grade 4 CRS occurred. Neurotoxicity was rare.5
“We find this study promising,” Madduri said. “We look forward to more long-term follow-up data, and a second portion of this study has fully accrued.” The dose for the phase II portion of the trial will be 0.75 × 106 CAR T cells/kg.5 In addition, the phase III CARTITUDE-4 trial (NCT04181827) has been initiated and is comparing JNJ-4528 against 2 regimens comprising pomalidomide and dexamethasone plus either daratumumab or bortezomib (FIGURE).
LEGEND-2 Follow-Up
LCAR-B38M, an identical CAR to JNJ-4528, was examined in China in the phase I LEGEND-2 trial, whose results were also discussed at the meeting.7 Earlier results presented in 2018 had demonstrated efficacy and manageable safety in 57 enrolled patients with relapsed/refractory multiple myeloma.6
Patients received a median dose of 0.5 × 106 cells/kg (range, 0.07-2.1 × 106) of LCAR-B38M as 3 separate infusions. The results demonstrated an ORR of 88%, with 74% of patients having a complete response (CR).7
The median PFS was 28.2 months for patients who attained CR versus 3.2 months in those without CR.7 MRD-negative CR was achieved in 68% of patients, and the median OS had not yet been reached at 25 months in the subset of patients with CR. Rates of very good partial response (VGPR) and partial response (PR) were 4% and 11%, respectively.7
Peak levels of LCAR-B38M (≥1 × 104 copies/μg genomic DNA) were observed in most patients with available blood samples for analysis, and 5 patients showed CAR persistence at 10 months.7
Adverse events (AEs) of pyrexia, thrombocytopenia, leukopenia, and anemia were among the most common toxicities. Ninety percent of the patients in the study experienced CRS, with a median onset of 9 days; 46% of patients with CRS received tocilizumab (Actemra). One patient experienced neurotoxicity, with manifestations including aphasia.
The results of the LEGEND-2 study led to the PRIME (PRIority MEdicines) designation of LCAR-B38M (JNJ-4528) by the European Medicines Agency.8 This decision, announced in a press release in April 2019, will facilitate production of the drug. According to the presentation at ASH, investigators have initiated the phase II CARTIFAN-1 confirmatory trial (NCT03758417) in China.7
CRB-402
The ongoing phase I CRB-402 study examining the next-generation anti-BCMA CAR T-cell therapy bb21217—which uses the lentiviral CAR T design of idecabtagene vicleucel (bb2121) combined with the PI3K inhibitor bb007 during ex vivo culture to create a more persistent and potent CAR product—showed promise in heavily pretreated patients with multiple myeloma.9 The trial followed an initial dose-escalation design, with patients treated with 150 × 106 (n = 12), 300 × 106 (n = 6), or 450 × 106 CAR+ T cells (n = 6).
“Overall, early and deep responses were observed in some patients,” Nina Shah, MD, associate professor of medicine at the University of California, San Francisco, and an investigator on the trial, said in an interview.
As of 2019, 83% of the patients were reported to have an objective response, with most patients also achieving MRD-negative status. The rate of CR or stringent complete remission (sCR) was 33%, and the VGPR rate was 50%.9
Twenty-five patients experienced CRS, and most were treated with tocilizumab; a few patients needed corticosteroids. At the highest dose, 1 person died from a CRS event and neurotoxicity. Nine patients experienced neurotoxicity, with 2 having a grade 3 event and 1 having a grade 4 event.9
Bispecific Anti-BCMA/CD38 CAR
A trial conducted in China introduced BM38, a new bispecific CAR T-cell therapy featuring tandem anti-BCMA and anti-CD38 single-chain variable fragments, as well as 4-1BB and CD3ζ signaling domains. The study produced an ORR of 90.1% in 22 patients and a rate of bone marrow MRD-negative status of 81.8%. Twelve patients achieved sCR, 2 had VGPR, 5 had PR, and 1 had a minor response. The PFS rate at 9 months was 78.9%, and the median PFS was not reached.10
Over 90% of study participants experienced CRS, with 22.7% of patients experiencing grade ≥3 CRS. No cases of neurotoxicity were observed. The incidence of hepatic AEs was 13.6%, and 1 patient experienced nephrotoxicity. Hematologic AEs were present in nearly every patient. Given the promising data, more studies of BM38 CAR T cells are expected. Investigators will follow the participants in this particular study for 2 years.10
Ongoing CAR T Studies In another ongoing study conducted in the United States, 10 patients with relapsed/refractory multiple myeloma received anti-BCMA CAR T cells, either alone or in combination with anti CD19 CAR T cells, following lymphodepletion with cyclophosphamide and fludarabine. Early results showed an acceptable safety profile and high response rates. The administered dose for both products, 5 × 108 CAR+ cells, was divided into 3 doses (10%, 30%, and 60%). The rationale for the CAR T-cell combination stems from the potential for relapse mediated by BCMA-negative/ CD19+ myeloma cells remaining after anti-BCMA CAR therapy alone.11
The PRIME trial, which is evaluating the P-BCMA-101 CAR T-cell product, is currently enrolling participants for the phase II portion. Phase I of the trial previously demonstrated efficacy and safety for P-BCMA-101 in patients with relapsed/refractory multiple myeloma. According to investigators, the piggyBac DNA Modification System used to produce these CAR T cells is associated with several advantages compared with the viral vector–based systems normally used.12
Meta-Analysis of BCMA CAR T
Findings from a meta-analysis of patients treated with BCMA CAR T-cell therapies that was conducted at the University Medical Center Hamburg-Eppendorf in Germany were presented by Nico Gagelmann and his colleagues.13 The analysis consisted of 15 prospective studies involving 285 patients with relapsed/ refractory multiple myeloma who were treated with CAR T-cell therapies targeting BCMA. The analyzed patients had received an average of 7 prior lines of therapy. The results showed an ORR of 82% (95% CI, 74%-88%) and a CR rate of 36% (95% CI, 24%-50%). The percentage of responsive patients with MRD-negative status was 77% (95% CI, 67%-85%), and the median PFS was 10 months.13
Most studies involved the use of fludarabine and cyclophosphamide for lymphodepletion prior to CAR T-cell administration. Fifteen percent of patients experienced grade 3/4 CRS (95% CI, 10%-23%), and 18% had neurotoxicity (95% CI, 10%-31%). Forty-five percent of responsive patients relapsed (95% CI, 27%-64%), and the authors rec-ommended larger studies with longer follow-up to evaluate the association between response and survival.13
T-Cell Engager
In a phase I study examining CC-93269, a bispecific antibody that bivalently binds BCMA on plasma cells and CD3 on T cells, each of the 30 patients enrolled had been treated with at least 3 other therapies.14,15 Doses of >6 mg to 10 mg resulted in an ORR of 88.9%, and the 10-mg dose led to an sCR of 44.4%.15 CRS affected 89.5% of the patients enrolled, with 57.9% experiencing grade 1 and 26.3% experiencing grade 2 CRS.14 One treatment-related death occurred; the patient had CRS, with a potential infection considered a contributing factor.14 Grade ≥3 AEs included neutropenia, anemia, infections, and thrombocytopenia.15
Future Promise
"The ability to target BCMA in multiple myeloma is one of the most attractive targets we have currently,” concluded Lonial. Referring specifically to belantamab mafodotin, he said “the ability to have something on the shelf to target BCMA is exciting, as patients with refractory multiple myeloma have few options, and those they do have are associated with significant AEs and short duration of response.”
References:
1. Cancer stat facts: myeloma. National Cancer Institute: Surveillance, Epidemiology, and End Results Program website. https://bit.ly/2RfmuPy. Accessed January 8, 2020.
2. Weisel K, Hopkins TG, Fecteau D, et al. DREAMM-3: a phase 3, open-label, randomized study to evaluate the efficacy and safety of belantamab mafodotin (GSK2857916) monotherapy compared with pomalidomide plus low-dose dexameth-asone (pom/dex) in participants with relapsed/refractory multiple myeloma (RRMM). Blood. 2019;134(suppl 1; abstr 1900). doi: 10.1182/blood-2019-129893.
3. Lonial S, Lee HC, Badros A, et al. Belantamab mafodotin for relapsed or refrac-tory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study [published online December 16, 2019]. Lancet Oncol. doi: 10.1016/S1470- 2045(19)30788-0.
4. Pivotal DREAMM-2 study demonstrated a clinically meaningful overall response rate with belantamab mafodotin (GSK2857916) for patients with relapsed/refractory multiple myeloma [news release]. London, United Kingdom: GlaxoSmithKline; December 16, 2019. bit.ly/2TeHXt0. Accessed January 9, 2020.
5. Madduri D, Usmani SZ, Jagannath S, et al. Results from CARTITUDE-1: a phase 1b/2 study of JNJ-4528, a CAR-T cell therapy directed against B-cell maturation anti-gen (BCMA), in patients with relapsed and/or refractory multiple myeloma (R/R MM). Blood. 2019;134(suppl 1; abstr 577). doi: 10.1182/blood-2019-121731.
6. Zhao W-H, Liu J, Wang B-Y, et al. Updated analysis of a phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B-cell maturation antigen, in patients with relapsed/refractory multiple myeloma. Blood. 2018;132(suppl 1; abstr 955). doi: 10.1182/blood-2018-99-110548.
7. Wang B-Y, Zhao W-H, Liu J, et al. Long-term follow-up of a phase 1, first-in-human open-label study of LCAR-B38M, a structurally differentiated chimeric antigen receptor T (CAR-T) cell therapy targeting B-cell maturation antigen (BCMA), in patients (pts) with relapsed/refractory multiple myeloma (RRMM). Blood. 2018;134(suppl 1; abstr 579). doi: 10.1182/blood-2019-124953.
8. European Medicines Agency grants Janssen PRIME designation for JNJ-68284528 (LCAR-B38M), an investigational BCMA CAR-T therapy discovered by Legend Biotech [news release]. Piscataway, NJ: Legend Biotech; April 3, 2019. bit.ly/39XhgiO. Accessed January 9, 2019.
9. Berdeja J, Alsina M, Shah N, et al. Updated results from an ongoing phase 1 clin-ical study of bb21217 anti-BCMA CAR T cell therapy. Blood. 2019;134(suppl 1;abstr 927). doi: 10.1182/blood-2019-126660.
10. Chenggong L, Heng M, Yu H, et al. A bispecific CAR-T cell therapy targeting BCMA and CD38 for relapsed/refractory multiple myeloma: updated results from a phase 1 dose-climbing trial. Blood. 2019;134(suppl 1; abstr 930). doi: 10.1182/ blood-2019-130340.
11. Garfall AL, Cohen AD, Lacey SF, et al. Combination anti-BCMA and anti-CD19 CAR T cells as consolidation of response to prior therapy in multiple myeloma. Blood. 2019;134(suppl 1; abstr 1863). doi: 10.1182/blood-2019-131515.
12. Costello CL, Gregory TK, Ali SA, et al. Phase 2 study of the response and safety of P-BCMA-101 CAR-T cells in patients with relapsed/refractory (r/r) multiple myeloma (MM) (PRIME). Blood. 2019;134(suppl 1; abstr 3184). doi: 10.1182/ blood-2019-129562.
13. Gagelmann N, Ayuk FA, Atanackovic D, Kroeger N. B cell maturation antigen-specific CAR T cells for relapsed or refractory multiple myeloma: a meta-analysis. Blood. 2019;134(suppl 1; abstr 3113). doi: 10.1182/blood-2019-121967.
14. Costa LJ, Wong SW, Bermúdez A, et al. First clinical study of the B-cell maturation antigen (BCMA) 2+1 T cell engager (TCE) CC-93269 in patients (Pts) with relapsed/ refractory multiple myeloma (RRMM): interim results of a phase 1 multicenter trial. Blood. 2019;134(suppl 1; abstr 143). doi: 10.1182/blood-2019-122895.
15. Early-phase trial suggests CC-93269 has activity in relapsed/refractory multi-ple myeloma. ASH Clinical News website. bit.ly/2t0r3nr. Updated January 15, 2019. Accessed January 16, 2019.
